Structural insight into the interaction of O‐acetylserine sulfhydrylase with competitive, peptidic inhibitors by saturation transfer difference‐NMR
FEBS Letters 590 (2016) 943–953 ª 2016 Federation of European Biochemical Societies
doi:10.1002/1873-3468.12126
O-acetylserine sulfhydrylase (OASS), the enzyme catalysing the last step of
cysteine biosynthesis in bacteria, is involved in antibiotic resistance and biofilm
formation. Since mammals lack OASS, it is a potential target for
antimicrobials. However, a limited number of inhibitors has been developed
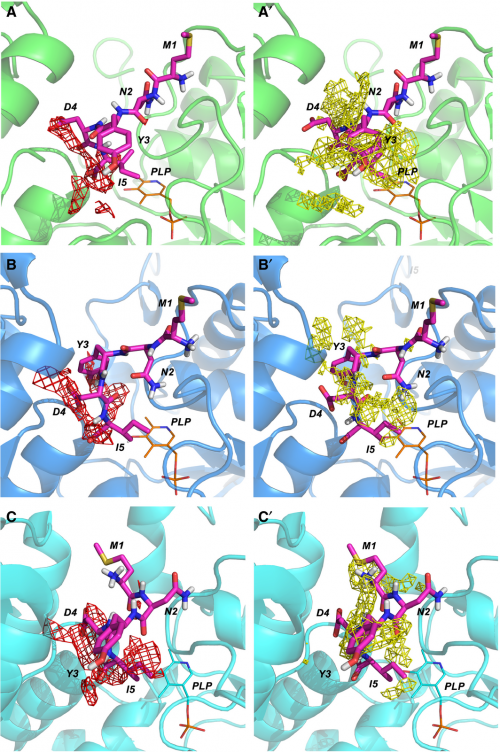
and crystallized in complex with OASS. STD-NMR was applied to study the
interaction of the inhibitory pentapeptide MNYDI with the CysK and CysM
OASS isozymes from Salmonella Typhimurium. Results are in excellent
agreement with docking and SAR studies and confirm the important role
played by the C-terminal Ile5 and the arylic moiety at P3 in dictating
affinity.
The pyridoxal 50-phosphate-dependent enzyme, O-acetylserine
sulfhydrylase (OASS), catalyses the last step of
the reductive sulfate assimilation pathway and has
been identified in bacteria, protozoa, mycobacteria
and plants. Different isoforms of OASS are
differentially expressed depending on growth conditions,
with at least two isoforms being identified
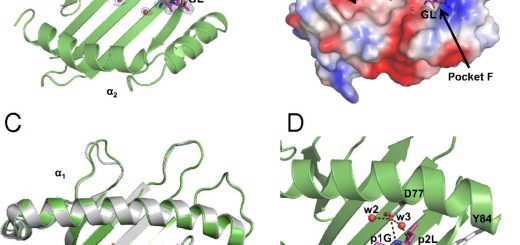
for each organism. Most proteobacteria,
including Salmonella enterica serovar Typhimurium
and Haemophilus influenzae, express the CysK and
CysM isoforms, that share a very similar three-dimensional
structure and a 70% sequence identity for residues
in the active site. Both isozymes can
synthesize cysteine from O-acetylserine and bisulfide
but CysM, owing to a larger active site, can
also use thiosulfate as nucleophile. was removed and protein purity was higher than 98%. The
specific activity was comparable to that of previous preparations




comments