A novel synthetic approach to the racemic Neuraminidase inhibitor Peramivir
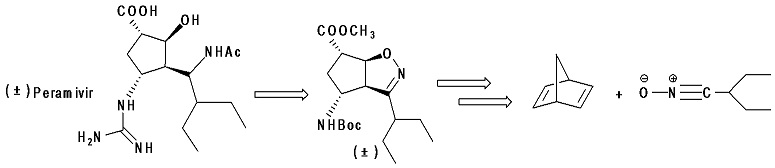
 An advanced intermediate in the synthesis of the racemic Neuraminidase inhibitor Peramivir was synthesised
An advanced intermediate in the synthesis of the racemic Neuraminidase inhibitor Peramivir was synthesised
in a new and versatile manner starting from a stereoselective 1,3-dipolar cycloaddition reaction
between the nitrile oxide deriving from 2-ethylbutanal and the commercially available and inexpensive
bicyclo[2.2.1]hepta-2,5-diene. The reaction mainly afforded the exo-isoxazolino-norbornene derivative
from which the oxidative cleavage of the carbonecarbon double bond followed by subsequent dehydration
led to the corresponding anhydride intermediate. Amines and alcohols were used as nucleophiles for
opening the anhydride, with amines providing the better results. Both the monoesteremonoacid and the
monoesteremonoamide were transformed into the monoesteremonoamino intermediate fromwhich the
synthesis continued using previously published methods. In the best protocol, the total yield of this key
intermediate was increased up to 17% from bicyclo[2.2.1]hepta-2,5-diene.
Tetrahedron 72 (2016) 7975e7981




comments