Structure-activity relationships of beta-hairpin mimics as modulators of amyloid beta-peptide aggregation
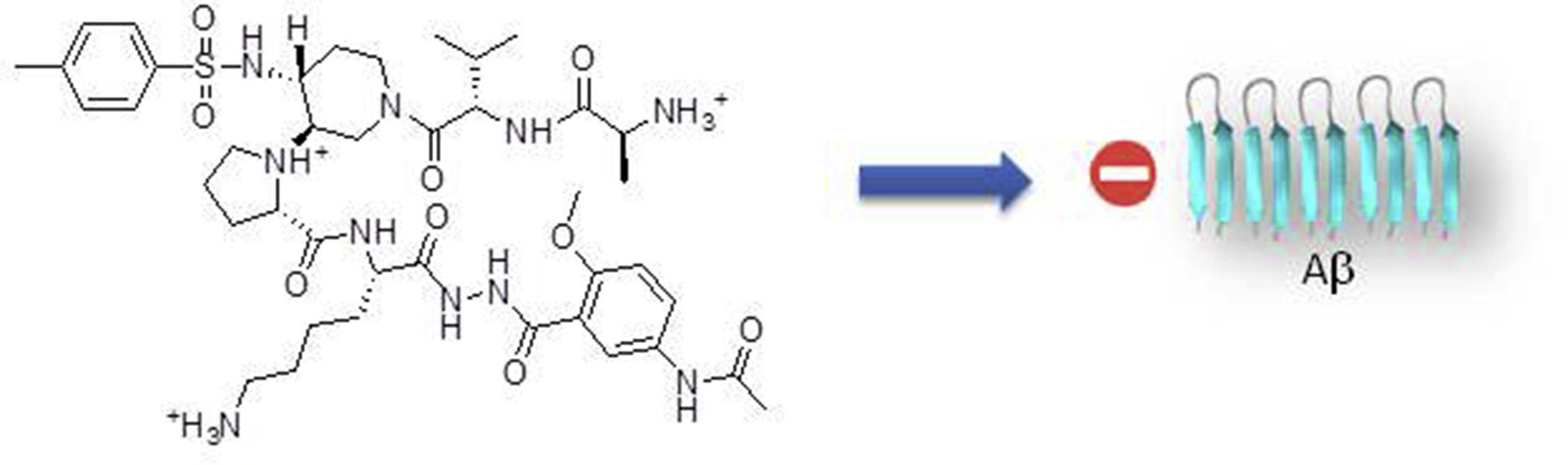
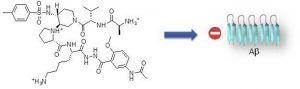 Aggregation of amyloid proteins is currently involved in more than 20 serious human diseases that are
Aggregation of amyloid proteins is currently involved in more than 20 serious human diseases that are
actually untreated, such as Alzheimer’s disease (AD). Despite many efforts made to target the amyloid
cascade in AD, finding an aggregation inhibiting compound and especially modulating early oligomerization
remains a relevant and challenging strategy.We report herein the first examples of small and nonpeptide
mimics of acyclic beta-hairpins, showing an ability to delay the fibrillization of amyloid-b (Ab1-
42) peptide and deeply modify its early oligomerization process. Modifications providing better druggability
properties such as increased hydrophilicity and reduced peptidic character were performed. We
also demonstrate that an appropriate balance between flexibility and stability of the b-hairpin must be
reached to adapt to the different shape of the various aggregated forms of the amyloid peptide. This
strategy can be investigated to target other challenging amyloid proteins.
https://doi.org/10.1016/j.ejmech.2018.05.018




comments