On‑resin multicomponent 1,3‑dipolar cycloaddition of cyclopentanone–proline enamines and sulfonylazides as an efficient tool for the synthesis of amidino depsipeptide mimics
Depsipeptides are biologically active peptide derivatives that possess a high therapeutic interest. The development of depsipeptide
mimics characterized by a chemical diversity could lead to compounds with enhanced features and activity. In this
work, an on-resin multicomponent procedure for the synthesis of amidino depsipeptide mimics is described. This approach
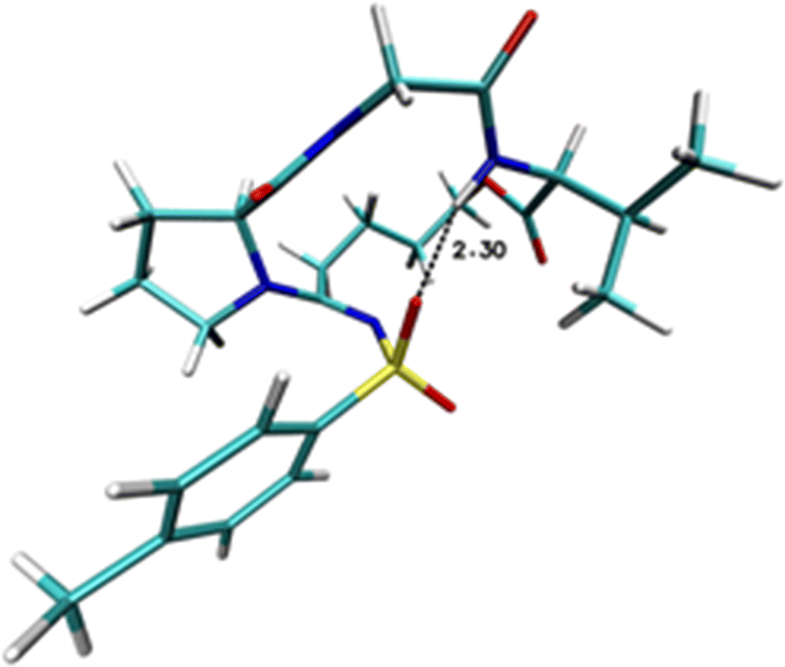
exploits a metal-free 1,3-dipolar cycloaddition of cyclopentanone–proline enamines and sulfonylazides. In this reaction,
the obtained primary cycloadduct undergoes a ring opening and molecular rearrangement giving access to a linear sulfonyl
amidine functionalized with both a peptide chain and a diazoalkane. The so-obtained diazo function “one pot” reacts with
the carboxylic group of N-Fmoc-protected amino acids leading to amidino depsipeptide mimics possessing a C4 aliphatic
chain. An important advantage of this procedure is the possibility to easily obtain amidino-functionalized derivatives that
are proteolytically stable peptide bond bioisosteres. Moreover, the conformational freedom given by the alkyl chain could
promote the obtainment of cyclic depsipeptide with a stabilized secondary structure as demonstrated with both in silico
calculations and experimental conformational studies. Finally, labeled depsipeptide mimics can be also synthesized using a
fluorescent sulfonylazide in the multicomponent reaction.
Amino Acids
https://doi.org/10.1007/s00726-019-02805-3
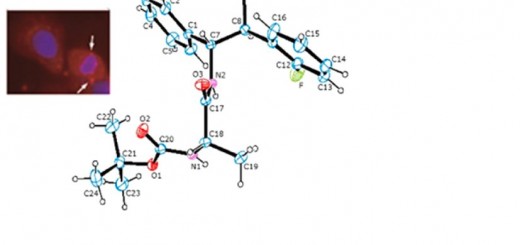




comments