Research
Macrocyclic ligands
Macrocyclic structures containing nitrogen atoms are widespread in nature. Indeed, polyazamacrocycles play fundamental role in biological processes as illustrated when considering porphyrins or related compounds. The study of polyazamacrocycles involves joint research from both organic synthetic and inorganic or organometallic chemists but, importantly, the utility of these ligands falls far beyond chemistry. For instance, polyazamacrocycles and their complexes have been extensively used in catalysis but have found an irreplaceable place in the field of medicine as therapeutic or diagnosis agents, as well as biological sensors.
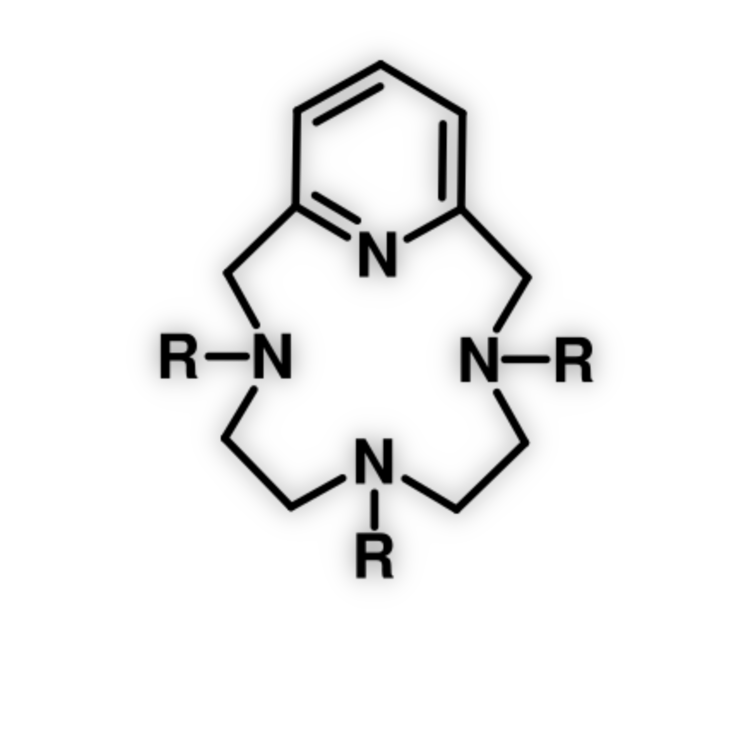
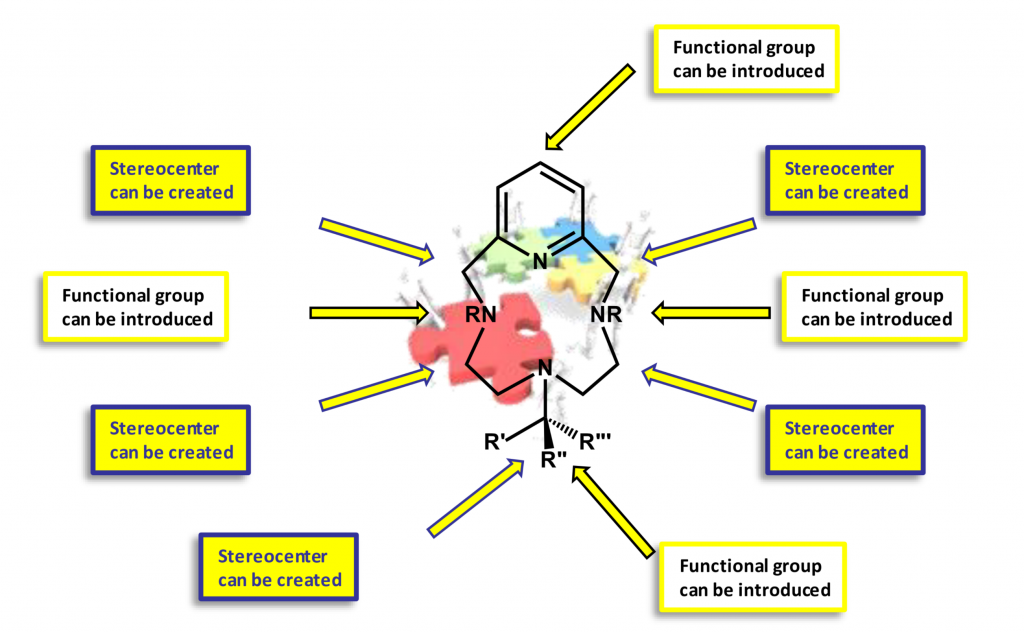
With virtually countless imaginable possibilities, there are several macrocyclic architectures that are ubiquitous. Among them, tetraaza-12-membered macrocycles, in which at least one of the N-atoms belongs to a pyridine ring, holds a privileged position. This fact could be attributed to the particular properties conferred to the macrocyclic ligand by the presence of the pyridine(s) ring(s) which are, subsequently transferred to the complex when coordinated. At first, the introduction of the pyridine ring allows a fast coordination due to the rigidity imposed by the pyridine ring. The corresponding complexes normally showed an increased thermodynamic and kinetic stability. We are interested in expanding the synthetic paths available for the synthesis of 12-membered macrocycles derived from pyclen (pyclen = 3,6,9-triaza-1(2,6)-pyridinacyclodecaphane).
Iron catalysts for oxidation reactions
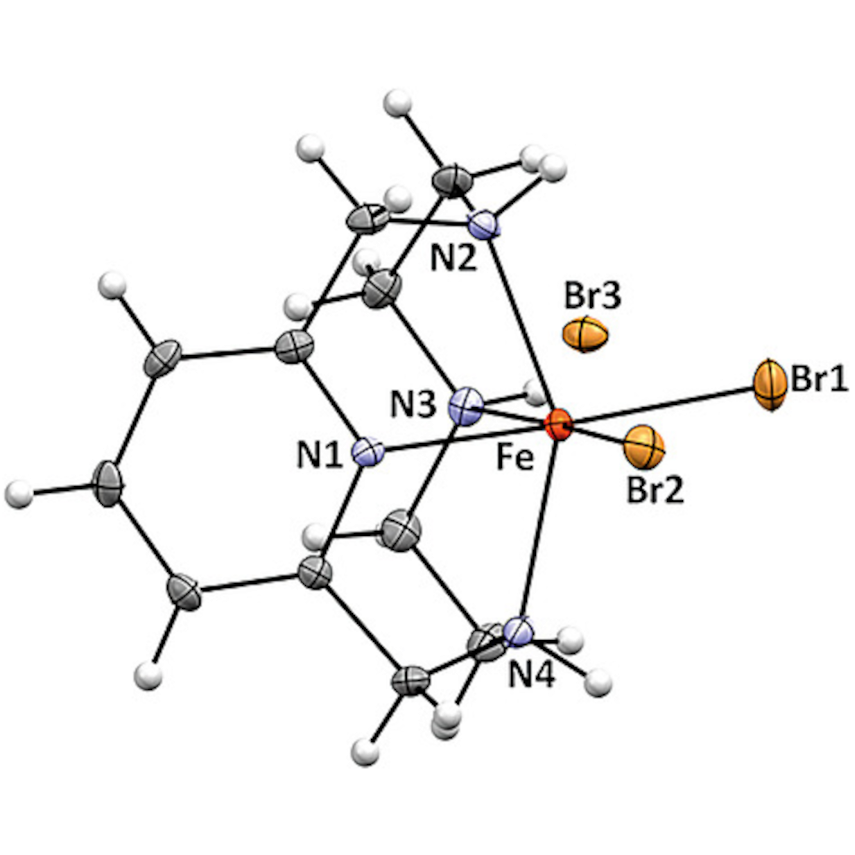
Iron, the most abundant transition metal on earth, and its complexes are knowing an increasing interest in organic synthesis. In particular, the field of iron–catalysed oxidation reactions is of great importance not only in synthetic organic chemistry, but also in biochemistry and industrial applications. We are interested in selective oxidation reactions using H₂O₂ as terminal oxidant promoted by iron(III) complexes in absence of any co-catalyst.

CO₂ valorization
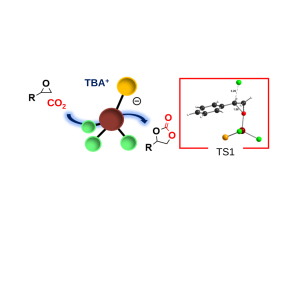
A mayor goal in current research is focused on developing methods to use pollutant CO₂ in the synthesis of high valued products. Among several alternatives, using CO₂ for the preparation of cyclic carbonates relies as one of the most interesting transformations. In this regard, we have recently disclosed that well-defined cationic Znᴵᴵ and Feᴵᴵᴵ – pyclen complexes were a competent catalyst for utilization of CO₂ in cycloaddition reactions with terminal epoxides.

Copper and silver in selective X-H functionalization
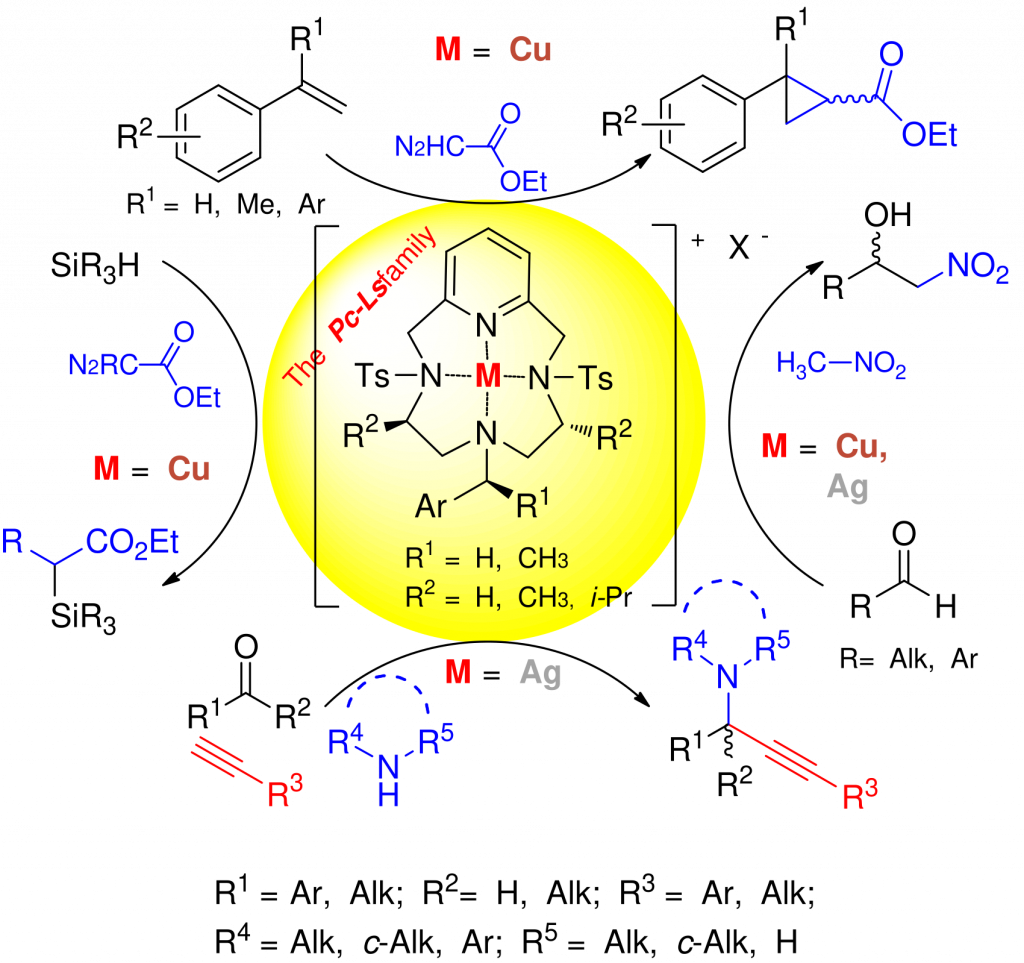
The selective functionalization of X–H bonds, particularly those with high bonding energies, constitutes appealing transformation with interest from synthetic and theoretical perspectives. We are interested in developing stereoselective functionalization of organic substates either via electrophilic metal–carbene intermediates generated from diazocompounds or via multicomponent reactions promoted by Lewis acid metal centers. Whenever possible, we make use of dielectric heating and/or Deep Eutectic Solvents (DES) as environmentally friendly “active” solvents.
Copper
- Cyclopropanation:
- Dalton Trans 2008, 4202.
- Dalton Trans 2013, 42, 2451.
- RSC Advances 2013, 3, 22199.
- Green Chemistry 2014, 16, 3202.
- Carbene X-H bond insertion:
- J. Organomet. Chem 2017, 835 , 1.
- Henry reaction:
- Appl. Organometal. Chem. 2011, 25, 824.
Silver
- Organometallic reactivity:
- Eur. J. Inorg. Chem 2015, 5089.
- A3 coupling:
- J. Org. Chem. 2014, 79, 7311.
- App. Organomet. Chem. 2022, DOI: 10.1002/aoc.6669
- Cycloisomerization:
- J. Org. Chem. 2014, 79, 3494.
- Eur. J. Org. Chem. 2020, 2592.
- Eur. J. Org. Chem. 2020, 3660.
- Henry reaction:
- RSC Advances 2016, 6, 97404.
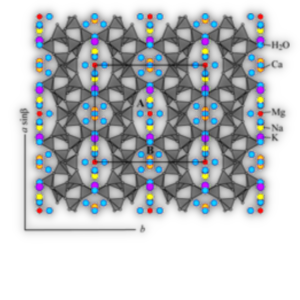
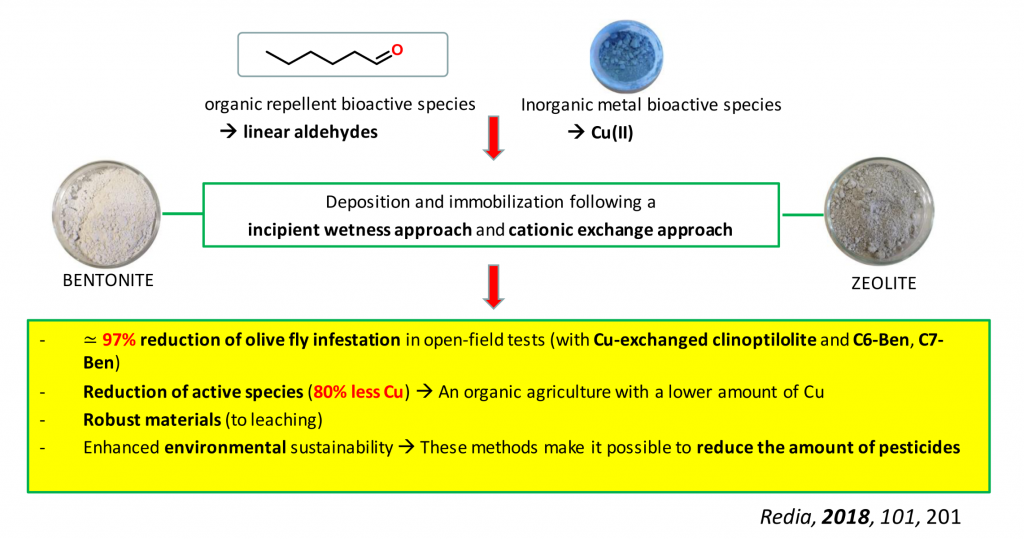
Inorganic solids for environmentally-friendly control methods
The implementation of novel environmentally–friendly control methods is attracting an ever–growing attention, especially after the recent ban on the use of the synthetic insecticide dimethoate. Inorganic solids, with high specific surface area and ion–exchange capacity, such as clays and zeolites, are promising alternatives for this application. We are interested in the synthesis of composite oxidic materials containing Cu(II) salts as biocides or organic repellant bioactive species such as linear aldehydes. Starting from two different solids support (bentonite and zeolite) we have decided to follow two different approaches: incipient wetness approach (with linear aldehydes – Hexanal and Heptanal) and cationic exchange approach (with Cu(II)), in order to obtain materials with limited environmental impact and that have a targeted and mild release of the bioactive principle. These materials proved to be easily prepared, cost effective, environmentally friendly, stable to rainwater leaching and led to a remarkable diminution in the use of bioactive species (and copper-containing ingredients) for on-field applications.