MOLECULAR MECHANISMS of ARRHYTHMIAS
Pathologies studied:
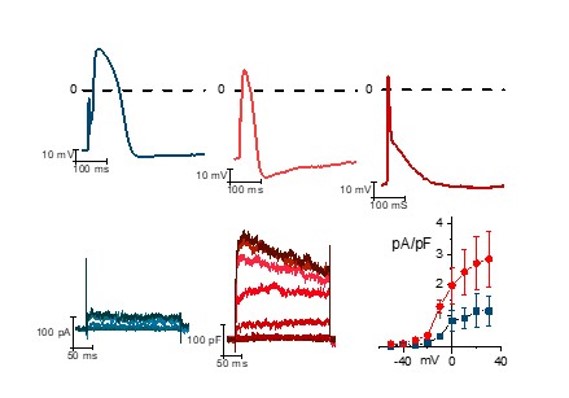
Familial form of Atrial Fibrillation (AF). (Benzoni et al 2020).
Atrial fibrillation in order to develop andsustain itsekf needs two conditions: 1) an appropriate substrate and 2) a trigger in the form of a focal ectopic activity. We study the molecular mechanism underlying the onset of AF in patients with monogenic and multigenic forms of atrial fibrilation. We use human induced pluripotents stem cells (hiPSC) generated from patients and healthy controls.
ELECTRO-MECHANICAL ALTERATIONS IN CAVEOLINOPATHIES
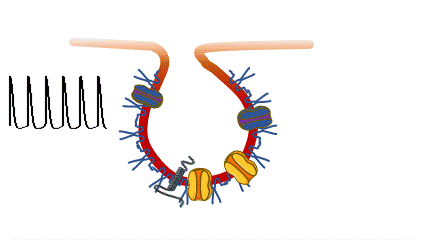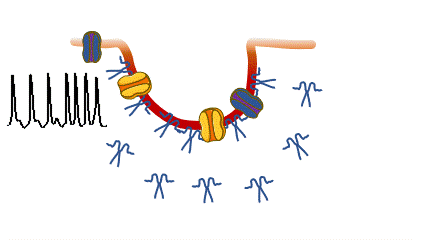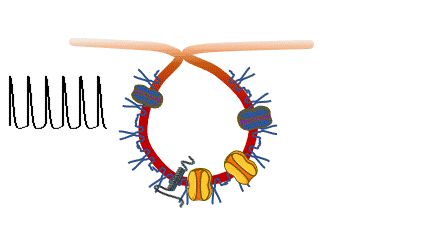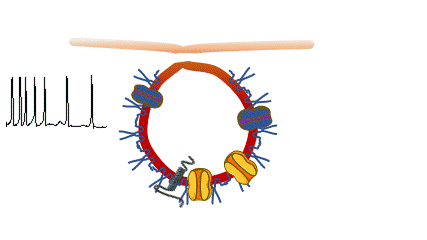
Caveolinopathies are a family of genetic disorders arising from mutations of the CAV3 gene (encoding for caveolin-3) that lead to some rare forms of muscular dystrophies, myopathies and arrhythmias during childhood. (Meraviglia et al 2018).
we are interested in understanding if and how various CAV3 mutations i patients with hyperCKemia, limb-girdle muscular distrophy, amd rippling muscle disease affect membrane fragility and cell excitability
STUDY OF THE CARDIAC ALTERATION ASSOCIATED WITH IDCCA
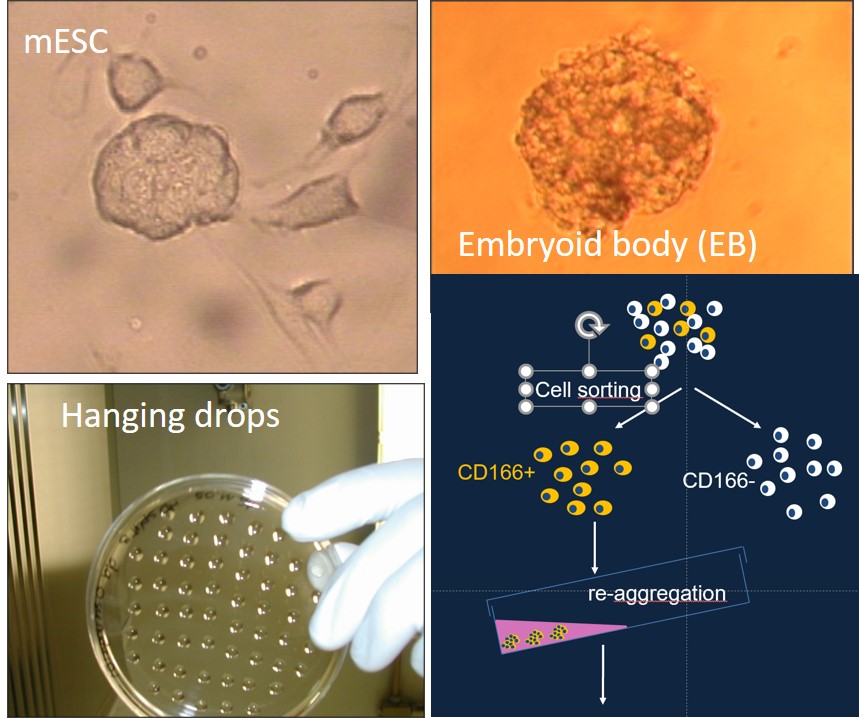
IDDCA is an autosomal recessive ultra-rare syndrome characterized by global developmental delay evolving into intellectual disability, cardiac abnormalities caused by mutations in the GNB5 gene
We use mouse Embryonic Stem Cells (mESC) KO for Gnb5 and human induced pluripotents stem cells obtained from patients with IDDCA harboring various mutations in the GNB5 gene, and induce the differentiation into pacemaker cells.
IDENTIFICATION OF UNCANONICAL SINOATRIAL NODE REGULATORS
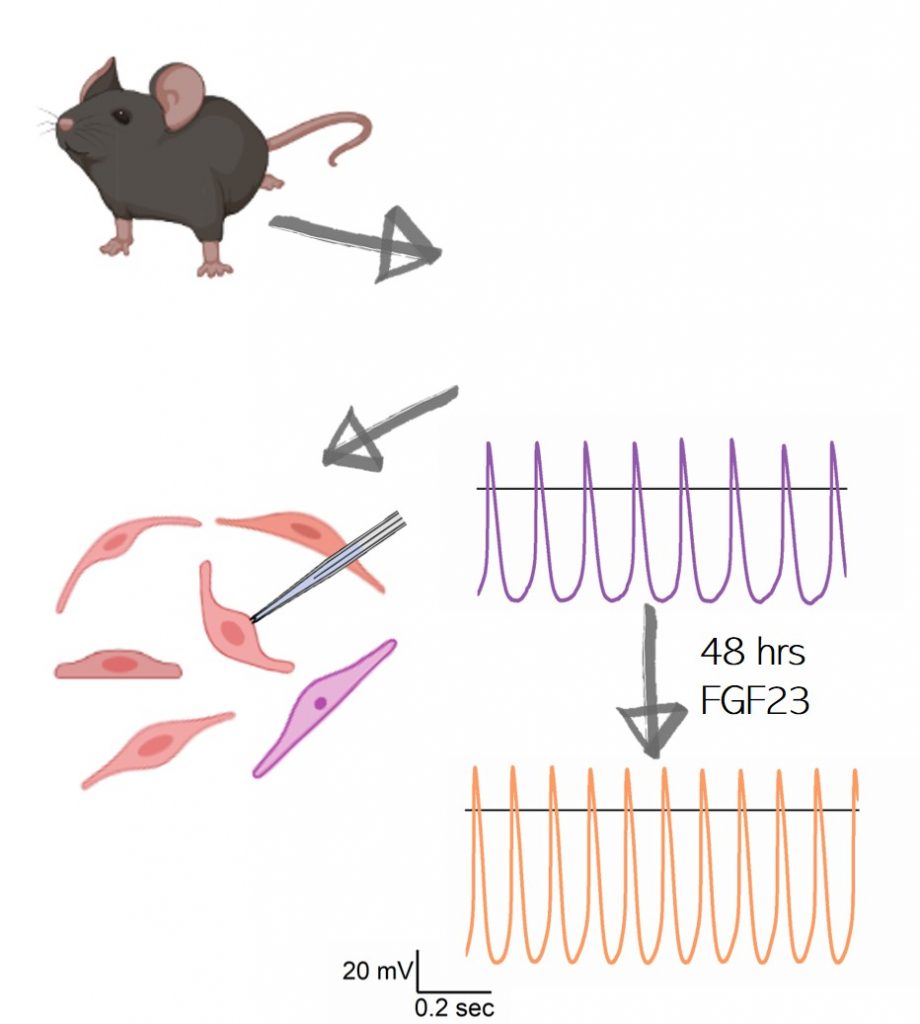
We investigate the effects of Sinoatrial node (SAN) modulators in the murine model and in human induced pluripotent stem cells-derived cardiomyocytes . Our studies focalize on the effects of new uncanonical modulators of SAN activity. In particular, we evaluate the impact of an hormone like molecule (FGF23) in the maintenance and modulation of the electrical activity of mouse SAN cells.
UNRAVELING THE DYSFUNCTIONAL ROLE OF MUTATIONS IN THE CARDIAC SODIUM CHANNEL SCN5A
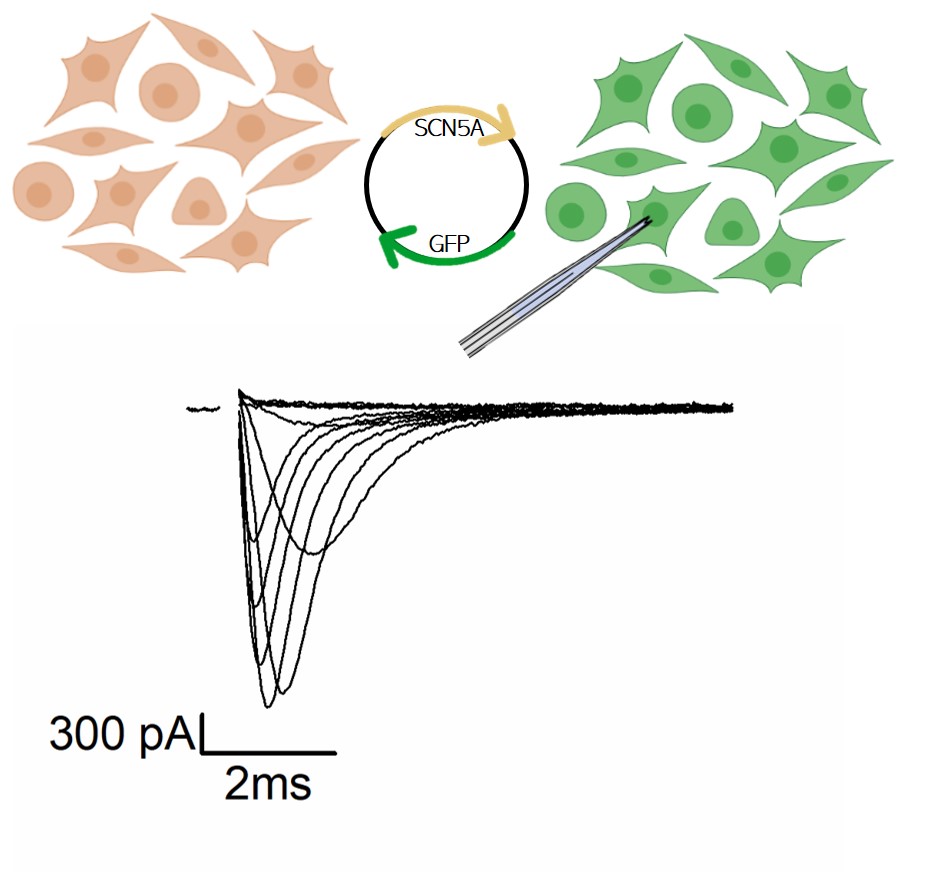
The aim of this project is to characterize the functional alterations caused by mutations in the cardiac voltage-gated sodium channel gene (SCN5A) found in patients that present different cardiac disease such as Brugada syndrome and Sick Sinus syndrome. We use the patch-clamp techniques in heterologous cell system expressing the WT and mutated channels
.