Synthesis of vaccine candidates
» Design and synthesis of oligosaccharides associated with pathogens and their mimics with potential immunological activity
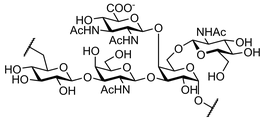
This research area is focused on the synthesis of fragments of selected saccharide antigens and their structural analogues
(such as carba-analogues, C-glycosides and zwitterionic derivatives). The overall goal is the construction of multivalent
systems by their conjugation to carrier proteins or nanoparticles, and the subsequent investigation of the biological and
immunological properties of the resulting conjugates in pursuit of the rational design of new carbohydrate-based vaccines.
For further informations on the project please visit glycovax.eu
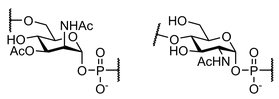
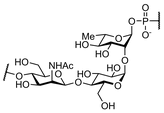
| Neisseria Meningitidis serotypes A and X | |
 |
 |
| Acinetobacter Baumanii ATCC 17961 | |
 |
 |
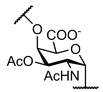
| Salmonella Typhi | |
|  |
| Streptococcus pneumoniae | |
|  |
Collaborations:
» Novartis Vaccines, Siena, Italy» Grazia Lombardi, DISCAFF, University of "Piemonte Orientale Amedeo Avogadro", Novara, Italy
» Ivan Zanoni, Department of Biotechnology and Biosciences, University of Milano-Bicocca, Milan, Italy
» Federica Compostella, Department of Medical Chemistry, Biochemistry and Biotechnology, University of Milan, Italy
» Dodi Safari, Eijkman Institute for Molecular Biology, Jakarta, Indonesia
» Jeroen Codee, Faculty of Science, Leiden Institute of Chemistry, Leiden, The Netherlands
Related publications:
» Fusari, Matteo et al. Bioorg. Med. Chem. 2015, 23 (23), 7439–7447 · DOI: 10.1016/j.bmc.2015.10.043» Fallarini, Silvia et al. ACS Infect. Dis. 2015, 1 (10), 487–496 · DOI: 10.1021/acsinfecdis.5b00071
» Morelli, Laura et al. Beilstein J. Org. Chem. 2014, 10, 2367-2376 · DOI: 10.3762/bjoc.10.247
» Gao, Qj et al. ACS Chem. Biol. 2013, 8 (11), 2561-2567 · DOI: 10.1021/cb400463u
» Ronchi, Paolo et al. J. Org. Chem. 2013, 78 (11), 5172-5183 · DOI: 10.1021/jo4001146
» Morelli, Laura and Lay, Luigi Arkivoc 2013, 166-184 · DOI: 10.3998/ark.5550190.0014.214
» Adamo, Roberto et al. ACS Chem. Biol. 2012, 7 (8), 1420-1428 · DOI: 10.1021/cb300221f
» Synthesis of metal-based nanoparticles for diagnostic and therapeutic applications
Among different polyvalent scaffolds capable of displaying multivalent interactions, gold nanoparticles (Au-NP) have enormous potential applications in biology and medicine. Au-NP can be coated with a strongly grafted monolayer of organic molecules (usually thiols) which can bear several biorecognition units. The ease of preparation, reproducibility and self-organized nature make Au-NP a quasiideal multivalent system for biological applications. Therefore, grafting synthetic saccharide fragments on gold nanoparticles would allow to compensate for their inherent poor immunogenicity, exploiting the property of multivalency to enhance the interaction of these nanosystems with specific antibodies.
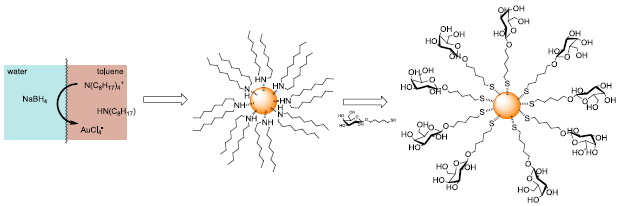
In addition, stabilized gold nanoparticles show interesting behaviour as computed tomography (CT) contrast agents owing to their high X-ray absorption coefficient, low toxicity and high biocompatibility. The performance of these materials, in terms of prolonged circulation time, biodistribution, enhanced renal clearance and little accumulation in reticuloendothelial system (RES) organs strongly depends on their particle size, shapes and the nature of stabilizing ligands. The elucidation of these main factors is crucial in order to obtain effective and reliable contrast agents at the nano-size.
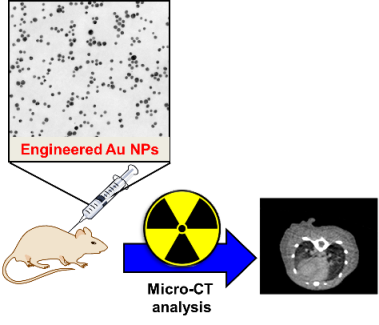
Collaborations:
» Laura Polito, CNR-ISTM, Milan, Italy» Paolo Scrimin, University of Padova, Italy
» Soledad Penadés, The Centre for Cooperative Research in Biomaterials-CIC biomaGUNE, San Sebastian, Spain
» Marco Marradi, Division of Materials, IK4-CIDETEC, San Sebastian, Spain
Related publications:
» Ramella, Daniela et al. Eur. J. Org. Chem. 2014, 59, 5915–592 · DOI: 10.1002/ejoc.201402701» Fallarini, Silvia et al. Nanoscale 2013, 5 (1), 390-400 · DOI: 10.1039/c2nr32338a
» Manea, Flavio et al. Adv. Mater. 2008, 20 (22), 4348-4352 · DOI: 10.1002/adma.200800737
Saccharides as tools in biomedicine
» Synthesis of multivalent conjugates of oligomers of N-acetylglucosamine as potential inhibitors of ligands of Mannose Binding Lectin (MBL) in a cerebral ischemia model
The project concerns the synthesis of glycodendrimers and glycodendrons decorated with oligomers of N-acetyl glucosamine that are able to inhibit the activity of Mannose Binding Lectin (MBL) in brain tissue affected by ischemia. MBL is a lectin that recognizes simple trimeric saccharide structures (based on mannose or N-acetylglucosamine) with low affinity, which can be increased substantially through their multivalent presentation on appropriate multifunctional scaffolds. The synthesized glycodendrimers should act as functional mimics of natural glycoconjugates and thereby inhibit the action of MBL in the brain affected by ischemic attack.
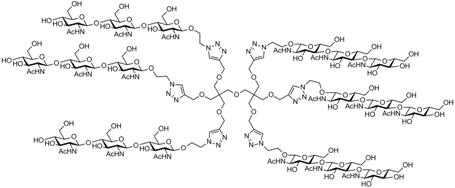
Collaborations:
» "Mario Negri" Institute, Milan, Italy» Javier Rojo, Instituto de Investigaciones QuÍmicas, Sevilla, Spain
» Valentin Wittmann, Fachbereich Chemie, Universität Konstanz, Germany
» Anna Bernardi, University of Milan, Italy
» Synthesis of molecular chimeras containing saccharide units as potential inhibitors of O-glucosaminidase (OGA)
The project involves the synthesis of molecular chimeras containing two chemical entities with distinct structures and functions: one unit is a potential inhibitor of caspases (a class of proteins highly involved in apoptosis), synthesized by the group of Prof. P. Seneci. The second entity is constituted by a saccharide unit potentially able to inhibit the activity of the enzyme O-glucosaminidase (OGA) and hence, in an indirect way, able to inhibit the hyperphosphorylation of TAU protein. According to recent studies, the TAU protein appears to be one of the main causes of neurodegenerative diseases, such as the Alzheimer's disease.
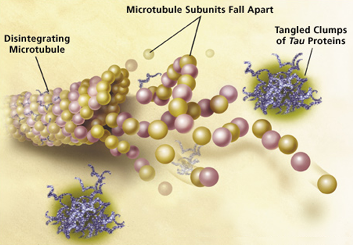
Collaborations:
» Pierfausto Seneci, CISI and University of Milan, Italy» Leonardo Manzoni, CNR-ISTM, Milan, Italy
Carbohydrates in catalysis
» Novel carbohydrate-based bifunctional organocatalysts for nucleophilic addition to nitroolefins and imines
Glucosamine has been selected as a cheap and readily available chiral scaffold for the synthesis of a series of novel enantiomerically pure bifunctional organocatalysts bearing a tertiary amino group in proximity to a (thio)urea group. The catalytic behaviour of these compounds, both as neutral and N-protonated species, was investigated using the addition of acetylacetone to β-nitrostyrene as a model reaction. Under optimized experimental conditions, chemical yields up to 93% and enantioselectivities up to 89% were obtained. Semiempirical (AM1) computational studies allowed to find a theoretical rationale for the chemical and stereochemical behaviour of the catalyst of choice. These catalysts were also preliminarily investigated as promoters in the addition of diethyl malonate to the N-Boc imine of benzaldehyde, affording the product in up to 81% ee.
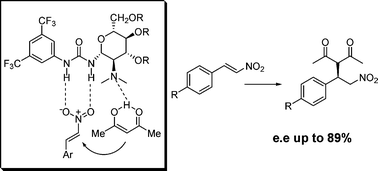
Collaborations:
» Maurizio Benaglia, University of Milan, ItalyRelated publications:
» Puglisi, Alessandra et al. Org. Biomol. Chem. 2011, 9 (9), 3295-3302 · DOI: 10.1039/c0ob01240h» Glycoporphyrins
Glycoporphyrins are generated by covalent attachment of saccharide units with a biocompatible porphyrin molecule. The crucial role of carbohydrates in vital ligand-receptor interaction and recognition phenomena makes sure that these compounds have several biological applications, such as in photodynamic therapy (PDT) for active targeting of membrane receptors. In addition, since metal-porphyrins are active in promoting nitrene and carbene transfer reactions, glycoporphyrin complexes with transition metal can be a new class of catalysts. Taking advantage of the chiral and hydrophilic nature of saccharide units, this class of compounds can be used either for asymmetric synthesis or to develop new sustainable water-soluble catalysts. We recently synthesized glycoporphyrin derivatives following two synthetic strategies: a) aromatic nucleophilic substitution using (pentafluoro)phenyl-porphyrin molecules and a sugar carrying an unprotected OH group; b) copper-catalyzed azide-alkyne cycloaddiction (CuAAC), starting from in situ generated tetra-(amino)phenyl-porphyrin and a sugar functionalised with a propargyl moiety. The corresponding Iron(III), Cobalt(II) and Ruthenium(II)-carbonyl complexes of the obtained glycoporphyrins were employed to perform a preliminary study on their catalytic activity, and on the photochemical properties of the free-base compounds.
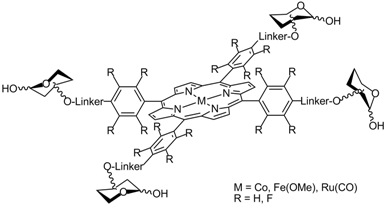
Collaborations:
» Emma Gallo, University of Milan, ItalyRelated publications:
» Tseberlidis, Giorgio et al. Organometallics 2015, 34 (15), 3774-3781 · DOI: 10.1021/acs.organomet.5b00436Flow chemistry
» Flow chemistry-mediated synthesis of biologically relevant oligosaccharides
The synthesis of complex carbohydrates remains a challenge for synthetic chemists. First, the monosaccharide intermediates required for the assembly of larger saccharide structures are usually prepared by multistep synthesis, involving tedious and time-consuming manipulation of multiple protecting groups. In addition, large amount of this precious starting material is often consumed for the identification of the best reaction conditions needed for the stereoselective formation of glycosidic bonds using traditional batch procedures, and their optimization and subsequent scale-up pose an additional hurdle. Continuous-flow microfluidic devices offer a well-engineered approach to meet some of these challenges, especially in terms of better control of reaction parameters. With the projects falling in this research area we intend to explore the high potential of this technology for applications in oligosaccharide synthesis using continuous-flow microreactors instead of round-bottomed flasks, typically employed in traditional batch procedures. A particular focus of our investigation is devoted to a systematic study of the glycosylation reaction carried out under microfluidic conditions, and to the synthesis of glycosyl phosphodiesters occurring in a variety of pathogen-associated carbohydrate antigens.
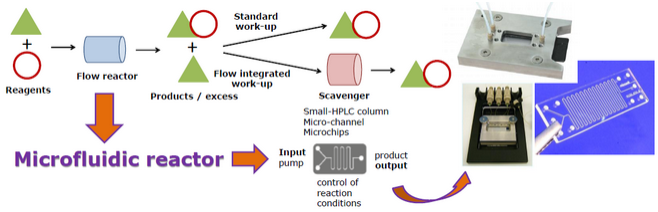
Related publications:
» Morelli, Laura et al. Beilstein J. Org. Chem. 2014, 10, 2367-2376 · DOI: 10.3762/bjoc.10.247» Cancogni, Damiano and Lay, Luigi Synlett 2014, 25 (20), 2873-2878 · DOI: 10.1055/s-0034-1379471