In inherently chiral molecules and molecular materials the same element is the source of both chirality and key functional properties that are thus strictly linked.
This can be achieved with a tailored torsion in the main molecular backbone with a energy barrier sufficiently high not to be overcome at room temperature, like in
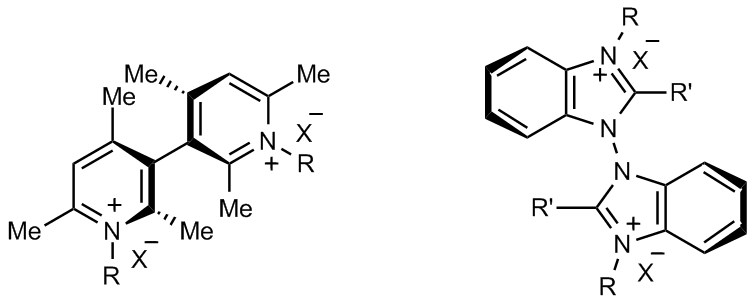
- molecules with atropisomeric (i.e. with hindered rotation between two moieties) biheteroaromatic cores, like bibenzothiophene, bithiophene, or biindole ones with oligothiophene wings (enabling electrodeposition of oligomer inherently chiral electrode surfaces) as well as bipyridinium or bibenzimidazolium cations with suitable anions, to be employed as inherently chiral ionic liquids or related additives;
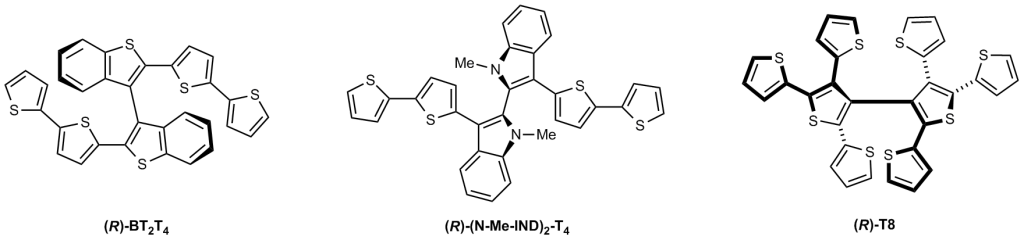
- helical molecules (like helicenes, among which particularly convenient are tetrathiahelicenes).
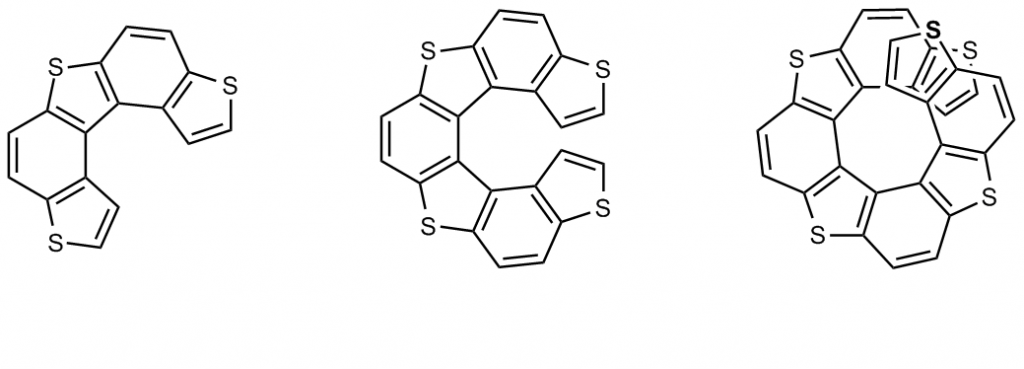
A second fundamental requirement is regioregularity (as in the above structures having a C2 symmetry axis), enabling oustanding chirality amplification and propagation in very stable helical or foldamer like macro- or supramolecular structures, accounted for by circular dichroism.