Starting from enantiopure inherently chiral monomers, enantiopure inherently chiral electrode surfaces can be obtained by fast electrooligomerization (or by deposition of chemically synthesized oligomers by physical methods like drop-casting; this can be useful particularly in cases where a single kind of oligomer rather than an oligomer mixture).
The (R)- or (S)- configurations of the starting monomers are entirely maintained in the corresponding electrodeposited films, as confirmed by nicely specular, sharp circular dichroism CD spectra.
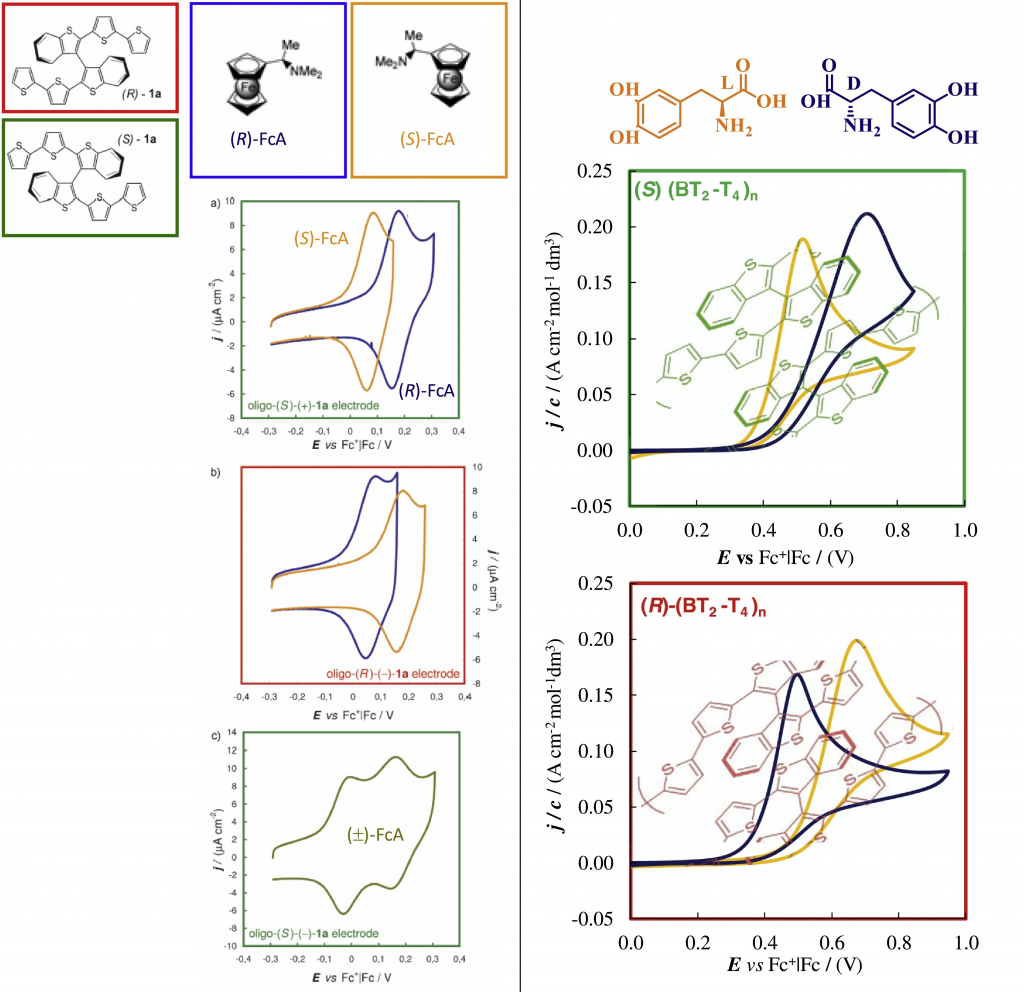
Such electrode surfaces show outstanding discrimination ability for the enantiomers of chiral probes, in terms of even very wide potential differences for cyclic voltammetry peaks. This feature is the most desirable one, enabling recognition of either enantiomer (combined with quantification from peak current).
Such property appears now to be of general character, since it holds with many combinations of inherently chiral selectors and chiral probes, on different supports and in different media. A given surface discriminates different probes and viceversa.
Different surfaces have been tested, originating from both atropisomeric or helical elements and deposited on many supports in different conditions; at the same time, tested chiral probes include molecules of quite different structure and reactivity, representative of different stereogenic elements. Discrimination is achieved with probes undergoing both reversible and irreversible processes, including oxidations and reductions.
Looking for a mechanistic justification, we are evaluating intermolecular selector/probe interactions involving (hetero)aromatic rings, heteroatoms as well the presence as cyclic oligomers of different dimensions.
However, a further possible fascinating justification has also very recently been suggested to us by a groundbreaking experiment now explained in the page Breaking News.