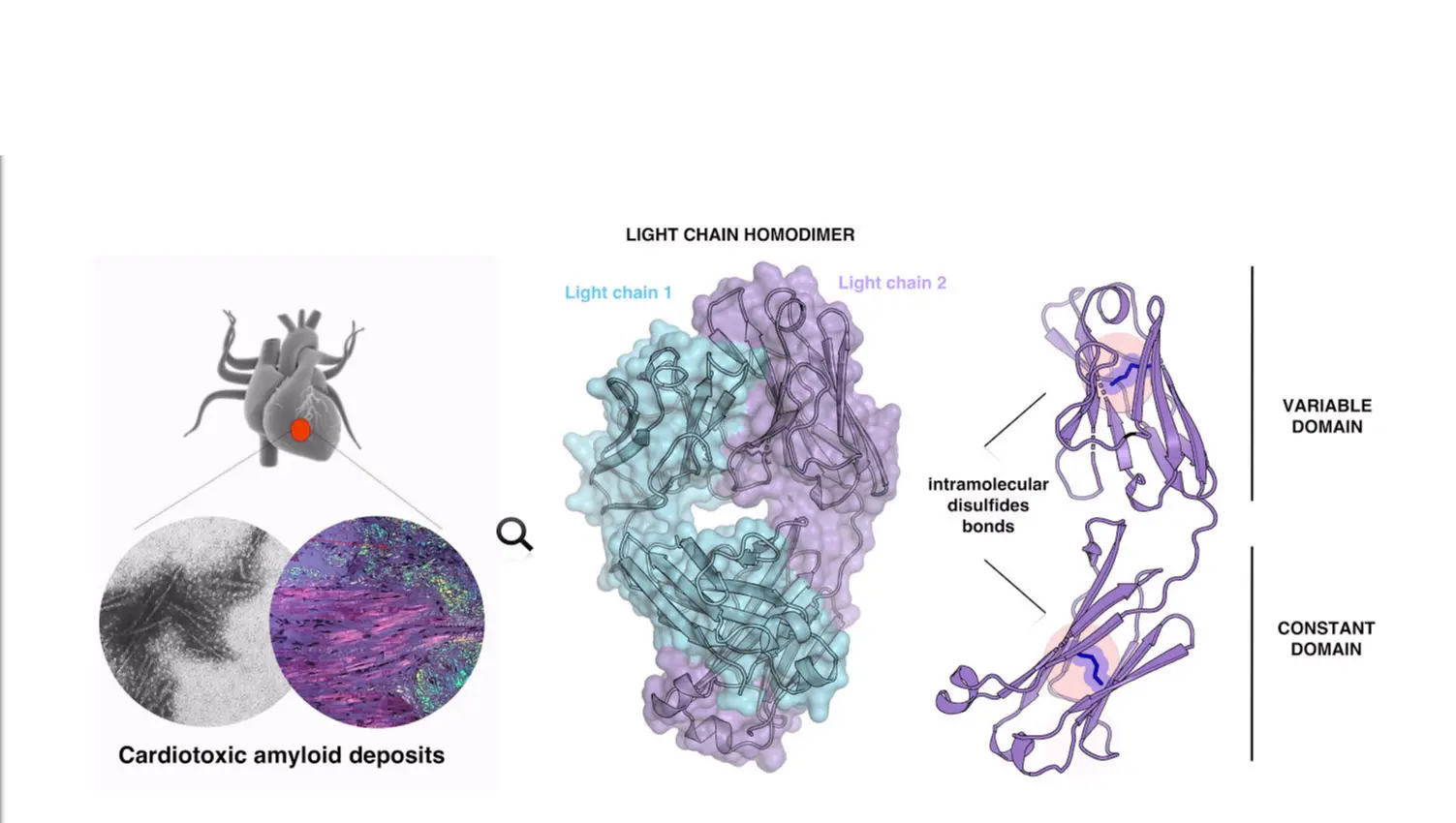

Systemic amyloidoses are protein-misfolding diseases characterised by widespread deposition of amyloid fibrils with severe dysfunction of the affected organs. Light chain (AL) amyloidosis is the most common systemic amyloidosis: fibrils originate from the aggregation of misfolding-prone immunoglobulin light chains (LCs); heart involvement is common and it is the main cause of death. Current therapies for AL amyloidosis are based on suppression of LCs production in the bone marrow by chemotherapy; however, severe heart involvement precludes the use of the most aggressive and most effective schemes. Solid clinical and experimental evidence, in AL as well as in other amyloidoses, indicates that cell and organ dysfunction is not only due to fibrils, but also to soluble, pre-fibrillar amyloidogenic precursors. In particular, soluble LCs that are cardiotoxic in patients were also shown to be toxicants for cardiac cells and model animals. However, the molecular features determining the ability of a subset of LCs to target the heart and to be toxic for cardiac cells are still largely undefined. Our aim is to understand the molecular properties determining the toxicity of specific LCs. In order to clarify this issue, we are working on a pool of toxic and non-toxic LCs and we characterise their structure, fold stability, flexibility and hydrophobicity. By site-directed mutagenesis and more in general by protein modification, we aim to pinpoint the toxic species and the biophysical and biochemical properties of LCs, which correlate with the toxicity in patients.
We use Cryo Electron Microscopy to determine the structure of amyloid fibrils ex-vivo, i.e. directly extracted from affected organs of patients.
We are developing in vitro inhibitors of aggregation and soluble cardiotoxicity by identifying molecules that can improve the biophysical properties of the amyloidogenic LCs.
Click here for the full list of publications on AL and other systemic amyloidoses.
Offline Website Maker