Since the discovery in the early 1970s of the photocatalytic water splitting on TiO2 electrodes by Fujishima and Honda, heterogeneous photocatalysis has drawn the interest of the scientific community as a very promising potential technology for the exploitation of sunlight to carry out chemically relevant processes for clean energy production, mainly in the form of hydrogen, as well as enabling to perform highly-efficient environmental remediation processes. In fact, exploitation of solar energy represents one of the most appealing developing technologies for the fulfilling of the humankind energetic demands due to the high availability and cleanness of the energy source, being also potentially very cheap.
Photocatalysis is generally defined as a process in which a substance, known as the photocatalyst, is being activated through the absorption of light radiation in order to initiate or modify the rate of a given chemical reaction, without being himself directly subject of any chemical transformation. Therefore, for a photocatalytic process to occur, it is necessary that a substance, usually a solid material, is capable of absorbing light of a given wavelength and utilize the acquired energy to promote the outcome of a chemical process whose occurrence would be impossible (or extremely slow) in its absence.
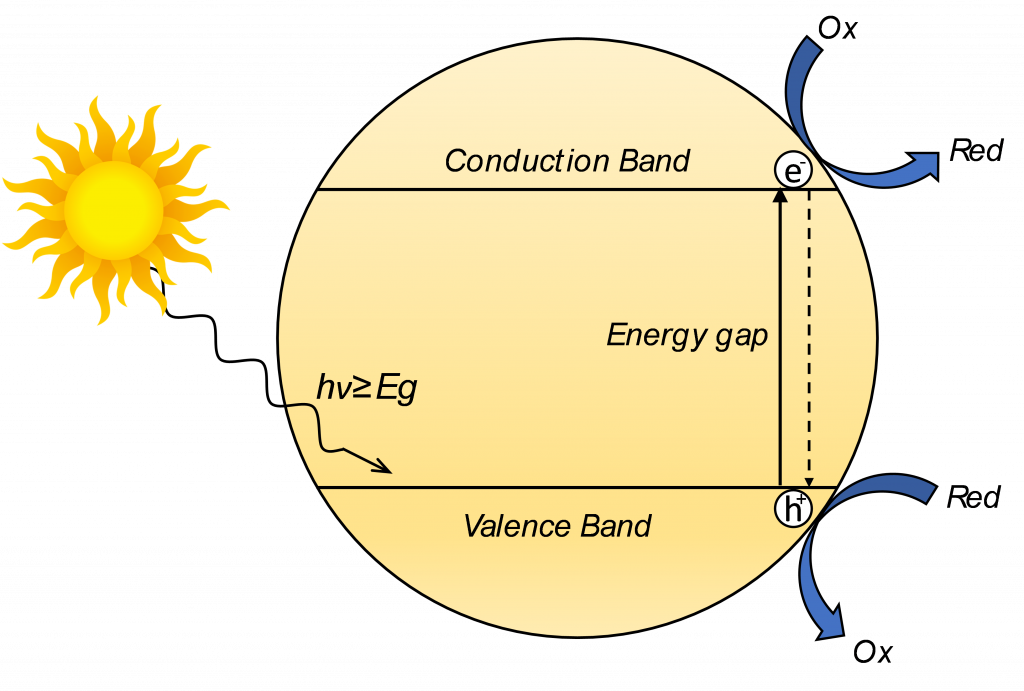
The most common substances employed and studied as photocatalysts are by far solid semiconductor materials, i.e. solid materials that, unlike metals, are characterized by an electronic structure in which a void energy region known as “energy band gap” is present between the top of the valence band, which is occupied by electrons, and the bottom of the conduction band, being vacant of electrons. Materials with such electronic structure are capable of being activated through absorption of photons whose energy is larger than the semiconductor energy band gap, resulting in the promotion of an electron from the top of the valence band into one of the energy states of the conduction band, simultaneously also leaving a hole in its valence band, resulting in an electronic excitation of the semiconductor material. The highly reactive species (electrons and holes) formed through such activation process are therefore readily available to be potentially transferred to reducible and oxidizable chemicals present at the surface of the semiconductor, thus resulting in the effective conversion of light energy into redox energy.
The performance of semiconductors as photocatalysts, however, are intrinsically restrained by a plethora of electron-hole recombination paths which are in competition with the desired charge transfer to the adsorbed substrates, resulting in the dissipation of the electronic excitation in the form of heat rather than promoting the outcome of a chemical process. Usually this phenomenon represents the major deactivation path of electronically excited semiconductors and may result in a significant suppression of the overall photocatalytic efficiency of the materials. Moreover, a semiconductor material can only interact with a finite set of substrates. In fact, for a semiconductor to be used as photocatalyst for a certain redox process, it is necessary that the redox potential energy of the conduction band is more negative than the redox potential of the oxidized substrate, while the valence band potential of the semiconductor should be more positive than the redox potential of the reduced substrate. Therefore, a semiconductor with a wider energy gap will be capable of promoting a wider range of chemical processes. However, the wider is the band gap, the higher is the energy required to provoke the electronic excitation of the semiconductor, resulting in a lower capability of the semiconductor material to utilize sunlight energy to carry out photocatalytic processes.
Our research focus
In our research group we focus on studying how we can improve the performance of semiconductor materials employed as photocatalysts by reducing electron-hole recombination rates using surface, morphological and bulk doping strategies.