
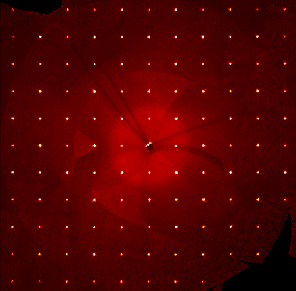
 With single crystal X-ray diffraction, we can experimentally determine from scratch the chemical structure of any molecule that can be crystallized. This technique is a sort of extremely powerful microscope, which allows us to quantitatively measure (i) the molecular connectivity and spatial arrangement of atoms in the solid-state and (ii) how molecules are arranged into stable three-dimensional structures. We can also follow the structural evolution of the material as a function of temperature, determining for example accurate estimates of thermal expansion coeffiecients, phase transition temperatures, and so on.
With single crystal X-ray diffraction, we can experimentally determine from scratch the chemical structure of any molecule that can be crystallized. This technique is a sort of extremely powerful microscope, which allows us to quantitatively measure (i) the molecular connectivity and spatial arrangement of atoms in the solid-state and (ii) how molecules are arranged into stable three-dimensional structures. We can also follow the structural evolution of the material as a function of temperature, determining for example accurate estimates of thermal expansion coeffiecients, phase transition temperatures, and so on.
Our focus is on small organic and metal-organic molecules (max ~ 50 atoms): we usually provide high-resolution (< 1 Angstrom) structures for research purposes. We preferentially operate down to the liquid nitrogen temperature (~ 100 K), but we can explore the phase diagram down to 20 K if needed. See also here for more information.
If you are interested in collaborating with us, please look here.
Relevant publications
Marta Papis, Sara Colombo, Leonardo Lo Presti, Giovanni Poli, Gianluigi Broggini, Julie Oble, Camilla Loro*. Palladium‐Catalyzed/Mn(OAc)3‐Mediated 1,2‐Diazidation and 1,2‐Acetoxy/Hydroxylation of N‐Allyl Sulfonamides. Adv. Synth. Catal. 2024, 366, 2477 – 2482. DOI: 10.1002/adsc.202400035
Marta Papis, Raffaella Bucci, Alessandro Contini, Maria Luisa Gelmi, Leonardo Lo Presti, Giovanni Poli, Gianluigi Broggini, Camilla Loro*. Phosphine-Catalyzed Domino Regio- and Stereo-Selective Hexamerization of 2-(Bromomethyl)acrylates to 1,2-Bis(cyclohexenyl)ethenyl Derivatives. Org. Lett. 2023, 25, 40, 7380–7384. DOI: 10.1021/acs.orglett.3c02836
Camilla Loro, Letizia Molteni, Marta Papis, Egle M. Beccalli, Donatella Nava, Leonardo Lo Presti, Stefano Brenna, Gioele Colombo, Francesca Foschi and Gianluigi Broggini*, Direct Synthesis of Fluorescent Oxazolo-phenoxazines by Copper-Catalyzed/Hypervalent Iodine(III)-Mediated Dimerization/Cyclization of 2-Benzylamino-phenols, J. Org. Chem. 2022, 87, 2, 1032–1042. DOI: 10.1021/acs.joc.1c02329
Sabrina Giofrè, Manfred Keller, Leonardo Lo Presti, Egle M. Beccalli* and Letizia Molteni, Switchable Oxidative Reactions of N -allyl-2-Aminophenols: Palladium-Catalyzed Alkoxyacyloxylation vs an Intramolecular Diels–Alder Reaction, Org. Lett. 2021, 23, 20, 7698–7702, DOI: 10.1021/acs.orglett.1c02539
Sabrina Giofrè, Letizia Molteni,Donatella Nava, Leonardo Lo Presti, Egle M. Beccalli*, Enantio‐ and Regioselective Palladium(II)‐Catalyzed Dioxygenation of (Aza)‐Alkenols, Angewandte Chemie International Edition 2021, 60(40):21723–21727. DOI: 10.1002/anie.202109312
Highly diastereoselective entry to chiral oxindole-based β-amino boronic acids and spiro derivatives. Org. Biomol. Chem., 2021,19, 7211-7216. DOI: 10.1039/D1OB01303C
Emanuele Bassini, Stefano Gazzotti, Filomena Sannio, Leonardo Lo Presti, Jacopo Sgrignani, Jean-Denis Docquier, Giovanni Grazioso, Alessandra Silvani*, Isonitrile-Based Multicomponent Synthesis of β-Amino Boronic Acids as β-Lactamase Inhibitors. Antibiotics 2020, 9(5), 249 (20pp). DOI: 10.3390/antibiotics9050249
Francesca Foschi, Camilla Loro, Roberto Sala, Oble Julie, Leonardo Lo Presti, Egle M. Beccalli, Giovanni Poli, Gianluigi Broggini*, Intramolecular Aminoazidation of Unactivated Terminal Alkenes by Palladium-Catalyzed Reactions with Hydrogen Peroxide as the Oxidant. Organic Letters 2020, 22, 4, 1402-1406, DOI: 10.1021/acs.orglett.0c00010
Stefano Gazzotti, Marco Manenti, Leonardo Lo Presti and Alessandra Silvani*: Allylation of isatin-derived N-Boc-hydrazones followed by Pd-catalyzed carboamination reaction: an entry to 3-spiro-pyrazolidyl-oxindoles. RSC Advances, 9 2019, 37788–3788, DOI: 10.1039/c9ra07712j
Elisa Bonandi, Paola Marzullo, Francesca Foschi, Dario Perdicchia, Leonardo Lo Presti, Maurizio Sironi, Stefano Pieraccini, Guido Gambacorta, Joern Saupe, Lisa Dalla Via, Daniele Passarella*, Stereodivergent Diversity‐Oriented Synthesis: Exploiting the Versatility of 2‐Piperidine Ethanol. European Journal of Organic Chemistry, 25, 2019, 4013–4019, DOI: 10.1002/ejoc.201900461
Sabrina Giofrè, Roberto Sala, Egle Maria Beccalli, Leonardo Lo Presti, Gianluigi Broggini*. Iodoamination of Alkenyl Sulfonamides by Potassium Iodide and Hydrogen Peroxide in Aqueous Medium. Helvetica Chimica Acta, 102, 2019, e190088, DOI: 10.1002/hlca.201900088
Sabrina Giofrè, Egle M. Beccalli *, Francesca Foschi, Concetta La Rosa, Leonardo Lo Presti, Michael S. Christodoulou. Chemo- and Regioselective Palladium(II)-Catalyzed Aminoarylation of N-Allylureas Providing 4-Arylmethyl Imidazolidinones. Synthesis 51, 2019, 3462-3470. DOI: 10.1055/s-0037-1611539
Luca Carlino, Michael Christodoulou, Valentina Restelli, Fabiana Caporuscio, Francesca Foschi, Marta S. Semrau, Elisa Costanzi, Annachiara Tinivella, Luca Pinzi, Leonardo Lo Presti, Roberto Battistutta, Paola Storici, Massimo Broggini, Daniele Passarella*: Structure–Activity Relationships of Hexahydrocyclopenta[c]quinoline Derivatives as Allosteric Inhibitors of CDK2 and EGFR. ChemMedChem 2018, 13, 2627-2634, DOI: 10.1002/cmdc.201800687
Cinzia Colombo*, Črtomir Podlipnik, Leonardo Lo Presti, Masahiro Niikura, Andrew J. Bennet, Anna Bernardi: Design and synthesis of constrained bicyclic molecules as candidate inhibitors of influenza A neuraminidase. PLoS ONE, 2018, 13(2) e0193623, 1 – 22, DOI: 10.1371/journal.pone.0193623
Giulia Rainoldi*, Fabio Begnini, Mariska de Munnik, Leonardo Lo Presti, Christophe M. L. Vande Velde, Romano Orru, Giordano Lesma, Eelco Ruijter, Alessandra Silvani: Sequential Multicomponent Strategy for the Diastereoselective Synthesis of Densely Functionalized Spirooxindole-Fused Thiazolidines. ACS Combinatorial Science, 2018, 20 (2), 98–105, DOI: 10.1021/acscombsci.7b00179
Lucia Tamborini, Federica Mastronardi, Leonardo Lo Presti, Birgitte Nielsen, Carlo De Micheli, Paola Conti, Andrea Pinto*: Synthesis of l‐Tricholomic Acid Analogues and Pharmacological Characterization at Ionotropic Glutamate Receptors. ChemistrySelect 2017, 2(31):10295-10299, DOI: 10.1002/slct.201702154
Michael S Christodoulou, Fabiana Caporuscio, Valentina Restelli, Luca Carlino, Giuseppe Cannazza, Elisa Costanzi, Cinzia Citti, Leonardo Lo Presti, Pasquale Pisani, Roberto Battistutta, Massimo Broggini, Daniele Passarella, Giulio Rastelli*: Probing an Allosteric Pocket of CDK2 with Small Molecules. ChemMedChem 01/2017; 12:33-41., DOI:10.1002/cmdc.201600474
Giulia Rainoldi, Matteo Faltracco, Leonardo Lo Presti, Alessandra Silvani*, Giordano Lesma: Highly diastereoselective entry to chiral spirooxindole-based 4-methyleneazetidines via formal [2+2] annulation reaction. Chemical Communications 08/2016; 52(77):11575-11578., DOI:10.1039/C6CC05838H
Michael S. Christodoulou*, Mikel Zarate, Francesca Ricci, Giovanna Damia, Stefano Pieraccini, Federico Dapiaggi, Maurizio Sironi, Leonardo Lo Presti, Aída Nelly García-Argáez, Lisa Dalla Via*, Daniele Passarella: 4-(1,2-diarylbut-1-en-1-yl)isobutyranilide derivatives as inhibitors of topoisomerase II. European Journal of Medicinal Chemistry 04/2016; 118:79-89., DOI:10.1016/j.ejmech.2016.03.090
Thomas C Eadsforth, Andrea Pinto, Rosaria Luciani, Lucia Tamborini, Gregorio Cullia, Carlo De Micheli, Luciana Marinelli, Sandro Cosconati, Ettore Novellino, Leonardo Lo Presti, Anabela Cordeiro-da-Silva, Paola Conti*, William N Hunter*, Maria Paola Costi*: Characterization of 2,4-Diamino-6-oxo-1,6-dihydropyrimidin-5-yl Ureido Based Inhibitors of Trypanosoma brucei FolD and Testing for Antiparasitic Activity. Journal of Medicinal Chemistry 08/2015; 58(20):7938–7948., DOI:10.1021/acs.jmedchem.5b00687
Lucia Tamborini*, Federica Mastronardi, Federica Dall'Oglio, Carlo De Micheli, Birgitte Nielsen, Leonardo Lo Presti, Paola Conti, Andrea Pinto: Synthesis of unusual isoxazoline containing β and γ-dipeptides as potential glutamate receptor ligands. MedChemComm 05/2015; 6(7):1260-1266., DOI:10.1039/C5MD00159E
Alessandro Ruffoni*, Alessandro Contini, Raffaella Soave, Leonardo Lo Presti, Irene Esposto, Irene Maffucci, Donatella Nava, Sara Pellegrino, Maria Luisa Gelmi, Francesca Clerici*: Model peptides containing the 3-sulfanyl-norbornene amino acid, a conformationally constrained cysteine analogue effective inducer of 310-helix secondary structures. RSC Advances 04/2015; 5(41):32643-32656., DOI:10.1039/C5RA03805G
Sara Pellegrino*, Alessandro Contini, Maria Luisa Gelmi, Leonardo Lo Presti, Raffaella Soave, Emanuela Erba: Asymmetric Modular Synthesis of a Semirigid Dipeptide Mimetic by Cascade Cycloaddition/Ring Rearrangement and Borohydride Reduction. The Journal of Organic Chemistry 03/2014; 79(7):3094–3102., DOI:10.1021/jo500237j
Roberta Ettari*, Lucia Tamborini, Ilenia C Angelo, Silvana Grasso, Tanja Schirmeister, Leonardo Lo Presti, Carlo De Micheli, Andrea Pinto*, Paola Conti: Development of Rhodesain Inhibitors with a 3‐Bromoisoxazoline Warhead. ChemMedChem 11/2013; 8(12):2070–2076., DOI:10.1002/cmdc.201300390
Lucia Tamborini, Andrea Pinto, Terry K Smith, Louise L Major, Maria C Iannuzzi, Sandro Cosconati, Luciana Marinelli, Ettore Novellino, Leonardo Lo Presti, Pui E Wong, Michael P Barrett, Carlo De Micheli, Paola Conti*: Synthesis and Biological Evaluation of CTP Synthetase Inhibitors as Potential Agents for the Treatment of African Trypanosomiasis. ChemMedChem 08/2012; 7(9):1623-34., DOI:10.1002/cmdc.201200304
Tam Luong Nguyen, Maria Rosaria Cera, Andrea Pinto, Leonardo Lo Presti, Ernest Hamel, Paola Conti, Rick Gussio, Peter De Wulf*: Evading Pgp Activity in Drug-Resistant Cancer Cells: A Structural and Functional Study of Antitubulin Furan Metotica Compounds. Molecular Cancer Therapeutics 03/2012; 11(5):1103-11., DOI:10.1158/1535-7163.MCT-11-1018
Lucia Tamborini*, Andrea Pinto, Paola Conti, Maddalena Gallanti, MC Iannuzzi, Leonardo Lo Presti, Carlo De Micheli: Regioselective Preparation of Functionalized Isoxazoline Derivatives as Key Intermediates for the Synthesis of Selective N-Methyl-D-aspartate Receptor Antagonists. Synthesis 04/2011;, DOI:10.1055/s-0030-1258477
Clelia Dallanoce*, Pietro Magrone, Carlo Matera, Leonardo Lo Presti, Marco De Amici, Loredana Riganti, Francesco Clementi, Cecilia Gotti, Carlo De Micheli: Synthesis of novel chiral Δ2-isoxazoline derivatives related to ABT-418 and estimation of their affinity at neuronal nicotinic acetylcholine receptor subtypes. European Journal of Medicinal Chemistry 09/2010; 45(12):5594-601., DOI:10.1016/j.ejmech.2010.09.009
Francesca Clerici*, Maria Luisa Gelmi, Raffaella Soave, Leonardo Lo Presti: Isothiazoles. Part 13. Synthesis of Sulfamic Esters, [1,2]Thiazete S,S-Dioxides, Benzo[e][1,2]thiazine S,S-Dioxides or Triazoles by Reaction of Isothiazole Dioxides with Sodium Azide. Tetrahedron 06/2002; 58(25):5173-5178., DOI:10.1016/S0040-4020(02)00445-3
