Physical Chemistry (Part B) – Prof. Gervasini
(Undergraduated program in Industrial Chemistry – Fall period)
Part A (Prof. E. Selli): The properties of gases: perfect gases and their mixtures, real gases, state equations. The first, second and third law of Thermodynamics: work, internal energy, heat, enthalpy, entropy, the Helmholtz and Gibbs energies, the fundamental equation, the chemical potential. Part B: Thermochemistry:standard enthalpy changes,standard enthalpies of formation,temperature dependence,calorimetric measurements. Phase equilibria: the phase rule, phase diagrams, phase equilibria in single component systems, the Clausius-Clapeyron equation, the vapour pressure, phase diagrams for binary systems Thermodynamics of solutions: ideal solutions, the Raoult’s and Henry’s laws, colligative properties of ideal solutions;non-ideal solutions,activity, activity coefficients and their determination. Reaction equilibria: equilibrium constants for gas phase reactions and reactions in solution, temperature dependence, simultaneous and independent reactions.
Numerical solution of problems of chemical thermodynamics: Part A and Part B (a.y. 2021/2022: Dr. Sebastiano Campisi)
Laboratory of Physical Chemystry – Prof. Gervasini
(Undergraduated program in Industrial Chemistry – Spring period)
CLICCA QUI PER UNA VISITA VIRTUALE DEL LABORATORIO
Theoretical concepts:
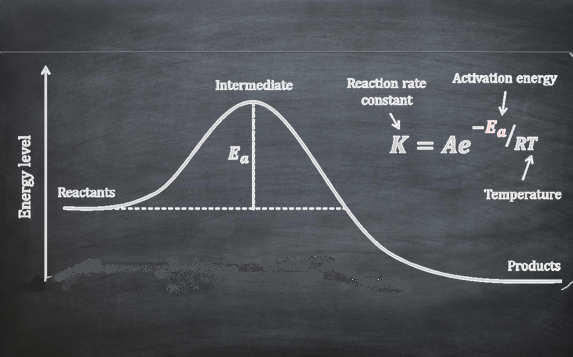
Reaction rate. Rate equation and rate constant. Order reaction and molecularity. Integrated kinetic equations for the principal order reactions (zero order-, first order-, second order-, and nth order- reactions). Half-time of reaction. Determination of reaction order. Parallel reactions. Opposing and equilibrium reactions. Consecutive reactions and the steady-state approximation. Dependence of the rate constant on temperature. Arrhenius equation. Eyring equation and transition state theory. Catalysed reactions. Homogeneous catalysis. Acid-base catalysis. Brönsted relations.
Practical experiments(Prof. Gervasini/Prof. Chiarello/Prof. Dozzi/Dr.Campisi):
isothermal reactions in batch reactors with homogeneous catalysts in liquid phase; gas-solid reaction under linearly increasing temperature.
Heterogeneous Catalysis – Prof. Gervasini
(Master in Industrial Chemistry – Spring period )
 Goals
The course aims to illustrate the heterogeneous catalysis in all its different aspects.
Catalysts and catalytic processes for the industrial and for environmental remediation processes, including photocatalysis, will be described.
Basic information on the design, preparation methods, bulk and surface catalyst properties, and development of industrial catalysts and catalytic processes will be illustrated.
Acquired Skills
At the end of the course, students will be able to understand the role of heterogeneous solids in given reactions, to know the main techniques suitable for characterize the surface and bulk properties of heterogeneous catalysts and to know the main surface reaction mechanisms by making laboratory tests. From the several illustrated case history, the students should grow a positive vision on the importance of heterogeneous catalysis in the industrial and environmental processes.
Course Content
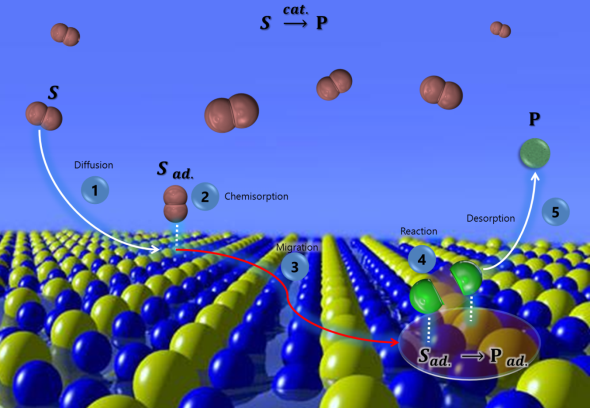
Definition of catalyst and catalytic reaction. Concepts of activity, selectivity, productivity, and yield of a catalyst. Kinetics and elemental steps: diffusion, adsorption, chemical reaction, and desorption. Catalytic action limited by external and internal diffusion. Development of industrial catalyst: design and preparation methods (unit operations for the preparation of bulk and supported catalysts, including the catalyst form); catalytic materials (active phase, supports, and promoters). Determination of bulk and surface catalyst properties by spectroscopic, thermal analysis, chemical, and X-ray based techniques. Catalyst ageing: deactivation, poisoning, and actions for the regeneration of spent catalysts.
Photocatalysis and photocatalyst: concepts and applications in several reactions for energy production and for environmental depollution.
Case histories: catalyst applications in the field of environmental problems resolution and sustainable chemistry.
Practical experiments: operations for the preparation of solid catalysts; determination of the specific surface area and porosity by nitrogen adsorption; determination of several catalyst properties with spectroscopic, thermal, microscopic methods; execution of catalytic and photo-catalytic reactions in batch and continuous reactors.
Suggested Prerequisites
Knowledge of thermodynamic and kinetic concepts.
Reference Material
J.M. Thomas, W.J. Thomas, Principles and Practice of Heterogeneous Catalysis, VCH, Weinheim, 1997 (ISBN 3-527-29239-X);
R.A. Sheldon, I. Arends, U. Hanefeld, Green Chemistry and Catalysis Wiley-VCH, Weinheim, 2007 (ISBN: 978-3-527-30715-9);
G. Rothenberg, Catalysis. Concepts and Green Applications, Wiley-VCH, Weinheim, 2008 (ISBN: 978-3-527-31824-7).
Assessment Method
In addition to an oral examination, which will cover all the topics treated in the classroom, the students will prepare a written Report on the experiments that will be shown during the laboratory practices.
For oral examination, students have to demonstrate to be able to treat any argument of the heterogeneous catalysis, among those treated during the course, with good mastery and to sustain a discussion on given catalytic aspects with the teachers.
Language of Instruction
English
Attendance Policy
Lessons are highly recommended and laboratory practices are mandatory.
Teaching Mode
Teaching mode includes both classic lessons and illustrations in the form of case histories of several articles among the most important ones appeared in recent years in the specific scientific literature.
Additional Information
Didactic material will be supplied by using the web site of the course on the ARIEL-UNIMI informatic platform.
Goals
The course aims to illustrate the heterogeneous catalysis in all its different aspects.
Catalysts and catalytic processes for the industrial and for environmental remediation processes, including photocatalysis, will be described.
Basic information on the design, preparation methods, bulk and surface catalyst properties, and development of industrial catalysts and catalytic processes will be illustrated.
Acquired Skills
At the end of the course, students will be able to understand the role of heterogeneous solids in given reactions, to know the main techniques suitable for characterize the surface and bulk properties of heterogeneous catalysts and to know the main surface reaction mechanisms by making laboratory tests. From the several illustrated case history, the students should grow a positive vision on the importance of heterogeneous catalysis in the industrial and environmental processes.
Course Content
Definition of catalyst and catalytic reaction. Concepts of activity, selectivity, productivity, and yield of a catalyst. Kinetics and elemental steps: diffusion, adsorption, chemical reaction, and desorption. Catalytic action limited by external and internal diffusion. Development of industrial catalyst: design and preparation methods (unit operations for the preparation of bulk and supported catalysts, including the catalyst form); catalytic materials (active phase, supports, and promoters). Determination of bulk and surface catalyst properties by spectroscopic, thermal analysis, chemical, and X-ray based techniques. Catalyst ageing: deactivation, poisoning, and actions for the regeneration of spent catalysts.
Photocatalysis and photocatalyst: concepts and applications in several reactions for energy production and for environmental depollution.
Case histories: catalyst applications in the field of environmental problems resolution and sustainable chemistry.
Practical experiments: operations for the preparation of solid catalysts; determination of the specific surface area and porosity by nitrogen adsorption; determination of several catalyst properties with spectroscopic, thermal, microscopic methods; execution of catalytic and photo-catalytic reactions in batch and continuous reactors.
Suggested Prerequisites
Knowledge of thermodynamic and kinetic concepts.
Reference Material
J.M. Thomas, W.J. Thomas, Principles and Practice of Heterogeneous Catalysis, VCH, Weinheim, 1997 (ISBN 3-527-29239-X);
R.A. Sheldon, I. Arends, U. Hanefeld, Green Chemistry and Catalysis Wiley-VCH, Weinheim, 2007 (ISBN: 978-3-527-30715-9);
G. Rothenberg, Catalysis. Concepts and Green Applications, Wiley-VCH, Weinheim, 2008 (ISBN: 978-3-527-31824-7).
Assessment Method
In addition to an oral examination, which will cover all the topics treated in the classroom, the students will prepare a written Report on the experiments that will be shown during the laboratory practices.
For oral examination, students have to demonstrate to be able to treat any argument of the heterogeneous catalysis, among those treated during the course, with good mastery and to sustain a discussion on given catalytic aspects with the teachers.
Language of Instruction
English
Attendance Policy
Lessons are highly recommended and laboratory practices are mandatory.
Teaching Mode
Teaching mode includes both classic lessons and illustrations in the form of case histories of several articles among the most important ones appeared in recent years in the specific scientific literature.
Additional Information
Didactic material will be supplied by using the web site of the course on the ARIEL-UNIMI informatic platform.Processi Catalitici (Catalytic processes)- Dr. Campisi
(Undergraduated program in Chimica, Chimica Industriale/Master in Scienze Chimiche- Fall period)
Il corso è erogato ESCLUSIVAMENTE in lingua ITALIANA.
Il nuovo programma per l’a.a. 2023/24 è riportato in basso
NUOVO! Programma del corso (a.a. 2023/24)
Modulo Zero: Introduzione e richiami di cinetica chimica (tot. 4 h)
Introduzione alla catalisi; Interazioni chimiche e ruolo nella catalisi; Reattività chimica: aspetti cinetici e termodinamici; Superfici di energia potenziale; Teorie della velocità di reazione.
Modulo A: Catalisi, sostenibilità ed economia circolare (tot. 6h)
A.1. Catalisi e industria (2h)
A.2. Catalisi sostenibile e circolare per la chimica verde (2h)
A.3. Catalisi Ambientale (2h)
Modulo B: Catalisi Omogenea (tot. 8h)
B.1. Catalisi omogenea (2h)
B.2. Catalisi acido-base specifica; Catalisi acido-base generale; (4h)
B.3. Case study 1: applicazioni industriali (2h)
Modulo C: Catalisi enzimatica (tot. 8h)
C.1. Catalisi Enzimatica (2 h) Struttura e classificazione degli enzimi;
C.2. Fondamenti di cinetica enzimatica (2h)
C.3. Strategie di immobilizzazione (2 h)
C.4. Case Study 2 (2h)
Modulo D: Catalisi Eterogenea (tot. 10 h)
D.1. Superficie chimica e reattività superficiale; (4h)
D.2. Adsorbimento e isoterme di adsorbimento (2h)
D.3. Nanocatalisi (2 h)
D.4. Case Study 3 (2h): Processi catalitici per la valorizzazione di biomasse
Prova orale in itinere (2h)
Modulo E: Catalisi Assistita (tot. 6h)
E.1. Principi di Elettrocatalisi (2 h)
E.2. Principi di Fotocatalisi (2 h)
E.3. Case study 4: Strategie elettro-catalitiche e fotocatalitiche per la conversione di CO2 (2h)
Modulo F: Chimica fisica dei materiali applicata alla catalisi (tot. 4h)
F.1. Aspetti chimico fisici della preparazione dei catalizzatori (2h)
F.2. Caratterizzazione chimico-fisica di materiali catalitici (2h)
Tavola rotonda: Esposizione dei lavori di gruppo (da svolgere entro il 29 febbraio 2024)
Modalità di esame:
L’esame potrà svolgersi in due forme (a scelta dello studente)
Esami parziali (altamente consigliato per studenti frequentanti): sono previsti due momenti intermedi per la valutazione della preparazione degli studenti. Una prima prova in itinere sarà programmata a conclusione dei moduli A-D e consisterà in un colloquio orale individuale che avrà come oggetto un singolo quesito su uno degli argomenti trattati nei suddetti moduli. La seconda prova in itinere sarà svolta alla fine del corso e consisterà in un lavoro di approfondimento che sarà presentato da studenti singolarmente o in gruppo e verrà autovalutato dalla classe sotto la guida del docente.
Esame totale: L’apprendimento da parte degli studenti verrà verificato con un esame orale che riguarderà tutti gli argomenti trattati. Sarà apprezzato il lavoro di approfondimento su un tema a scelta dello studente che è stato presentato durante le lezioni.