beta-Hairpin mimics containing a piperidine– pyrrolidine scaffold modulate the beta-amyloid aggregation process preserving the monomer species
DOI: 10.1039/C6SC03176E
Alzheimer’s disease is a neurodegenerative disorder linked to oligomerization and fibrillization of amyloid
b peptides, with Ab1–42 being the most aggregative and neurotoxic one. We report herein the synthesis
and confo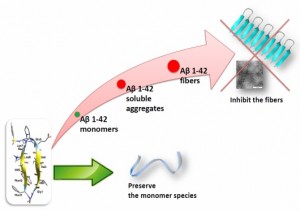 rmational analysis of Ab1–42-amyloid related b-hairpin peptidomimetics, built on a piperidine–
rmational analysis of Ab1–42-amyloid related b-hairpin peptidomimetics, built on a piperidine–
pyrrolidine semi rigid b-turn inducer and bearing two small recognition peptide sequences, designed on
oligomeric and fibril structures of Ab1–42. According to these peptide sequences, a stable b-hairpin or
a dynamic equilibrium between two possible architectures was observed. These original constructs are
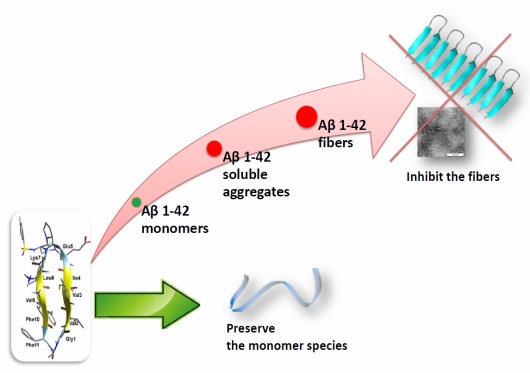
able to greatly delay the kinetics of Ab1–42 aggregation process as demonstrated by thioflavin-T
fluorescence, and transmission electron microscopy. Capillary electrophoresis indicates their ability to
preserve the monomer species, inhibiting the formation of toxic oligomers. Furthermore, compounds
protect against toxic effects of Ab on neuroblastoma cells even at substoichiometric concentrations. This
study is the first example of acyclic small b-hairpin mimics possessing such a highly efficient antiaggregation
activity. The protective effect is more pronounced than that observed with molecules which
have undergone clinical trials. The structural elements made in this study provide valuable insights in the
understanding of the aggregation process and insights to explore the design of novel acyclic b-hairpin
targeting other types of amyloid-forming proteins.




comments