Conformational switch andmultiple supramolecularstructures of a newly identified self-assembling protein-mimetic peptide from Pseudomonas aeruginosa YeaZ protein
Protein-mimetic peptides (PMPs) are shorter sequences of self-assembling proteins, that represent remarkable building blocks for the generation of bioinspired functional supramolecular structures with multiple applications.
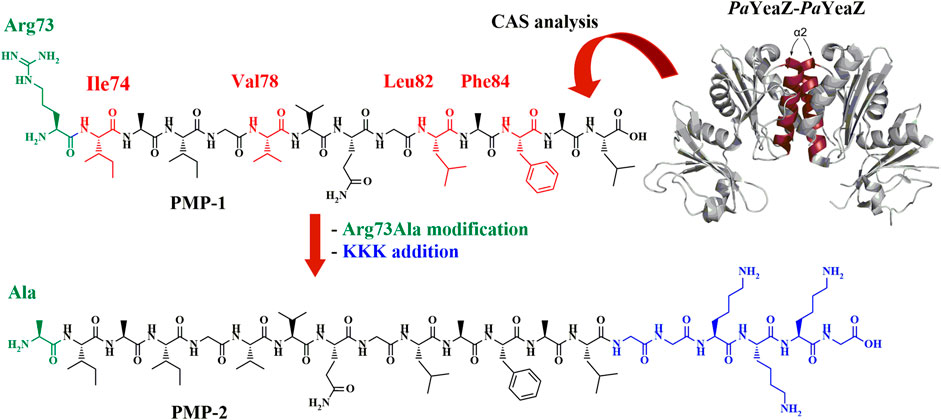
The identification of novel aminoacidic sequences that permit the access to valuable biocompatible materials is an attractive area of research. In this work, in silico analysis of the Pseudomonas aeruginosa YeaZ protein (PaYeaZ) led to the identification of a tetradecapeptide that represents the shortest sequence responsible for the YeaZ-YeaZ dimer formation. Based on its sequence, an innovative 20-meric peptide, called PMP-2, was designed, synthesized, and characterized in terms of secondary structure and self-assembly properties.
PMP-2 conserves a helical character and self-assembles into helical nanofibers in non-polar solvents (DMSO and trifluoroethanol), as well as in dilute (0.5 mM) aqueous solutions. In contrast, at higher concentrations (>2 mM) in water, a conformational transition from α-helix to β-sheet occurs, which is accompanied by the Protein-mimetic peptide aggregation into 2D-sheets
and formation supramolecular gel in aqueous environment. Our findings reveal a newly identified Protein-mimetic peptide that could turn as a promising candidate for future material applications.




comments