Innovative On-Resin and in Solution Peptidomimetics Synthesis via Metal-Free Photocatalytic Approach
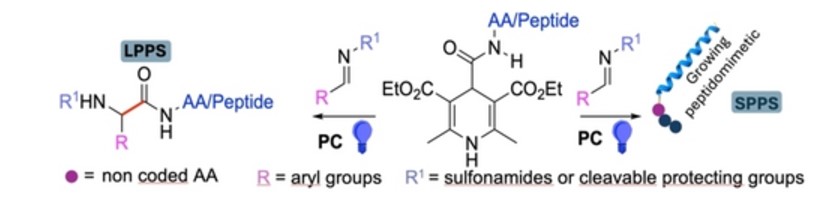
Now a days, peptidomimetics are widely studied, being useful tools in drug discovery and medicinal chemistry. The coupling between a carboxylic acid with an amine to form a peptide bond is the most common reaction to obtain peptides/peptidomimetics. In this work, we have investigated an innovative metal-free photoredox-catalyzed carbamoylation to form peptidomimetics thank stothereaction between dihydro pyridines functionalized with aminoacids (orpeptidesequences) and differently functionalized imines. As the organic photocatalyst, weused 4CzIPN, adonor-acceptor cyanoarene vastly used in photoredox catalysis. By e asily modulating the aminoacid (orpeptidesequence),which is directly attached to the dihydropyridine, we proposed this key-reaction as new valuable method toobtainp eptidomimetics, in situ building thenot-natural portion of the sequence. Moreover,we success fully employed this methodology in solid phase peptide
synthesis, both inserting the new photoredox-generated amino acidat the endor in the middleof the sequence. Peptides with different lengths and secondary structures were prepared, proving the successof this approach, eveninsterically hindered environment.Herein, o the best of our knowledge,we describe the first photocataly tic protocol which allows the building of the peptide back bone, with the possibility of simultaneously inserting an on-coded aminoacid in the sequence.
doi.org/10.1002/chem.202402790




comments