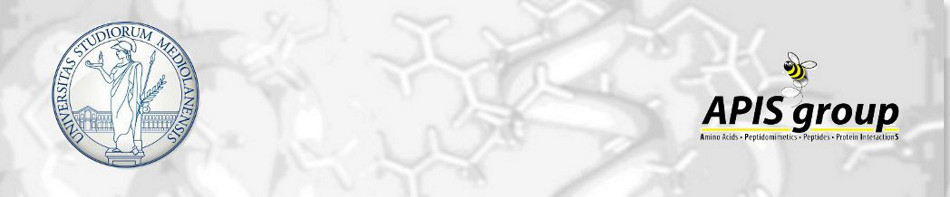
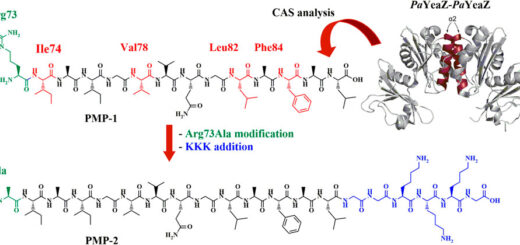
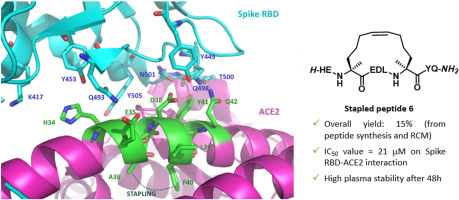
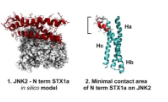
Tuning PFKFB3 Bisphosphatase Activity Through Allosteric Interference

The human inducible phospho-fructokinase bisphosphatase isoform 3, PFKFB3, is a crucial regulatory node in the cellular metabolism. The enzyme is an important modulator regulating the intracellular fructose-2,6-bisphosphate level. PFKFB3 is a bifunctional enzyme with an exceptionally high kinase to phosphatase ratio around 740:1. Its kinase activity can be directly inhibited by small molecules acting directly on the kinase active site. On the other hand, here we propose an innovative and indirect strategy for the modulation of PFKFB3 activity, achieved through allosteric bisphosphatase activation. A library of small peptides targeting an allosteric site was discovered and synthesized. The binding affinity was evaluated by microscale thermophoresis (MST). Furthermore, a LC-MS/MS analytical method for assessing the bisphosphatase activity of PFKFB3 was developed. The new method was applied for measuring the activation on bisphosphatase activity with the PFKFB3-binding peptides. The molecular mechanical connection between the newly discovered allosteric site to the bisphosphatase activity was also investigated using both experimental and computational methods.
https://doi.org/10.1038/s41598-019-56708-0




comments