Peptide modulators of Rac1/Tiam1 protein-protein interaction: An alternative approach for cardiovascular diseases
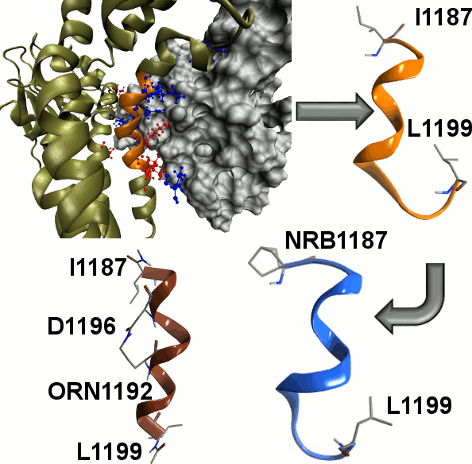
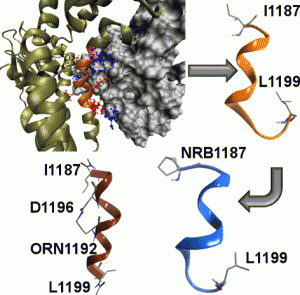 Rac1 GTPase interaction with guanine nucleotide exchange factor Tiam1 is involved in several cancer types and cardiovascular diseases. Although small molecules interfering with their proteinprotein interaction (PPI) were identified and studied, the ability of small peptides and peptide mimics acting as Rac1/Tiam1 PPI inhibitors has not been yet explored. Using computational alanine scanning (CAS), the “hot” interfacial residues have been determined allowing the design of a small library of putative PPI inhibitors. In particular, the insertion of an unnatural alpha, alpha disubstituted amino acid, that is norbornane amino acid, and the side chain stapling have been evaluated regarding both conformational stability and biological activity. REMD calculations and CD studies have indicated that one single norbornane amino acid at the N-terminus is not sufficient to stabilize the helix structure, while the side-chain stapling is a more efficient strategy.
Rac1 GTPase interaction with guanine nucleotide exchange factor Tiam1 is involved in several cancer types and cardiovascular diseases. Although small molecules interfering with their proteinprotein interaction (PPI) were identified and studied, the ability of small peptides and peptide mimics acting as Rac1/Tiam1 PPI inhibitors has not been yet explored. Using computational alanine scanning (CAS), the “hot” interfacial residues have been determined allowing the design of a small library of putative PPI inhibitors. In particular, the insertion of an unnatural alpha, alpha disubstituted amino acid, that is norbornane amino acid, and the side chain stapling have been evaluated regarding both conformational stability and biological activity. REMD calculations and CD studies have indicated that one single norbornane amino acid at the N-terminus is not sufficient to stabilize the helix structure, while the side-chain stapling is a more efficient strategy.
Furthermore, both engineered peptides have been found able to reduce Rac1-GTP levels in cultured human smooth muscle cells, while wild type sequence is not activ.
doi.org/10.1002/bip.23089




comments