Stereoselective Synthesis of α,α′-Dihydroxy-beta, beta′-diaryl-beta-amino Acids by Mannich-Like Condensation of Hydroarylamides
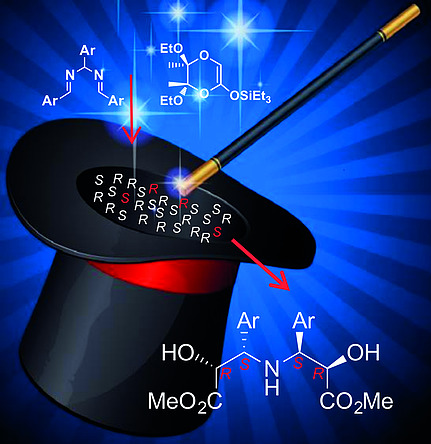
Dual α,α′-Dihydroxy-beta,beta’-beta-amino acids are highly interesting tools for several industrial applications. Nevertheless, only few derivatives are reported in the literature and knowledge concerning the substitution pattern as well as their enantioselective syntheses are lacking. Herein, we report on the preparation of enantiopure α,α′-dihydroxy-,′-diaryl–amino acid (dual) derivatives by an efficient Mannich-like condensation of hydroarylamides with 5,6-diethoxy-5,6-dimethyl-1,4-di oxan-2-one (triethylsilyl)ketene acetal. The synthetic protocol has been optimized affording the dual compounds in very good yields and with different aryl substitution patterns. Taking advantage of the “double stereodifferentiation” concept, a highly stereoselective reaction was performed: of the 16 possible isomers, only two diastereoisomers (d.r. up to 93:7) formed. Insights into the high stereocontrol of this condensation were given.
DOI: 10.1002/ejoc.201901325




comments