Successive crystal structure snapshots suggest the basis for MHC class I peptide loading and editing by tapasin
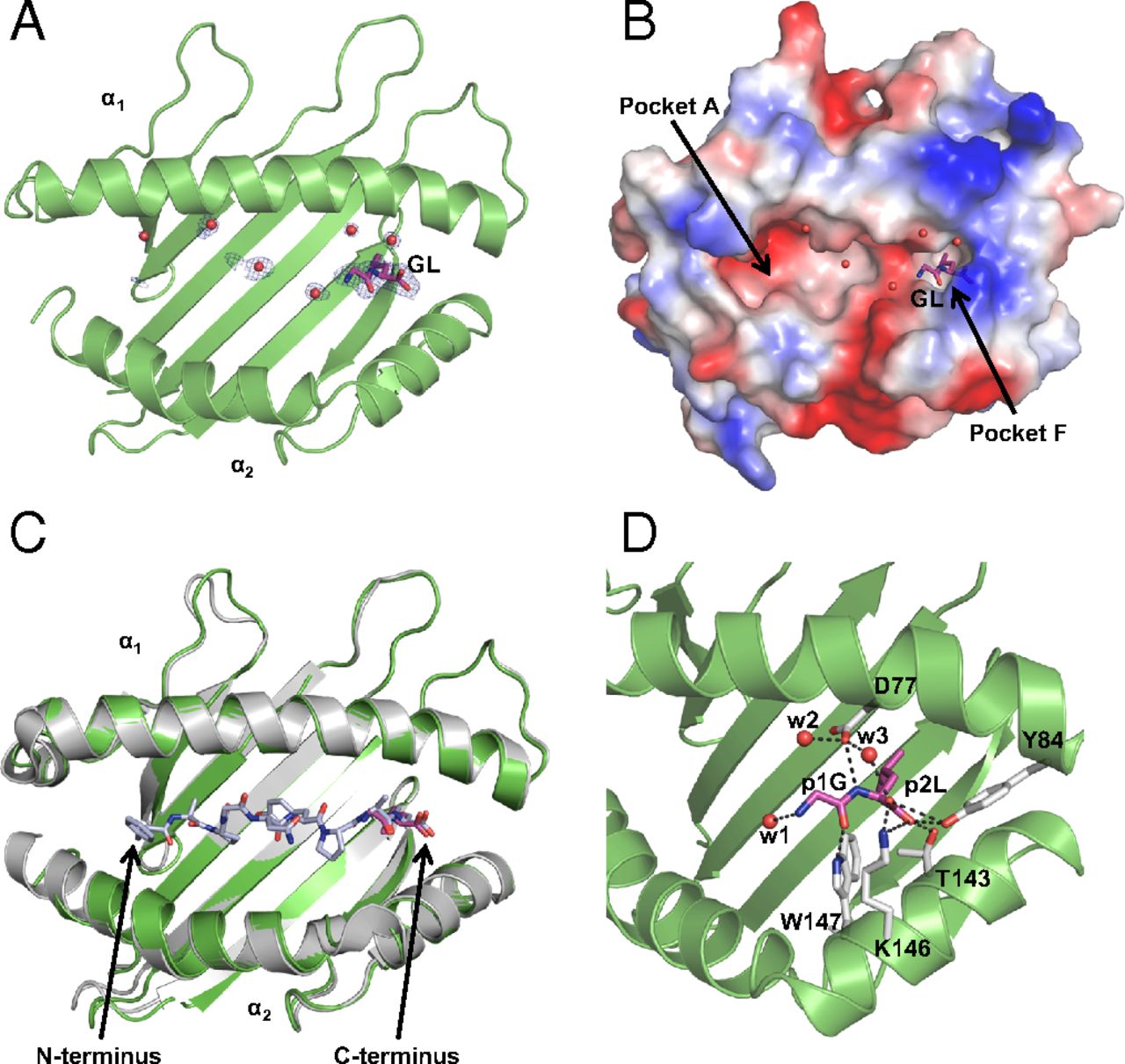
We have determined a suite of crystal structures of MHC-I molecules that provide insights in the molecular basis underlying peptide binding and selection by tapasin. We demonstrate that the scoop loop of tapasin binds in the F pocket of MHC-I molecules without affecting the conformation of the rest of the peptide binding cleft, which remains empty and peptide-receptive. Furthermore, the results of our study suggest that peptides may bind first with their N termini to the peptide binding cleft and are tested thereafter for their capacities to dislodge and replace the tapasin scoop loop from the F pocket. Mutation studies revealed the importance of specific residues within the scoop loop of tapasin, with significant differences for selection between different MHC-I alleles.
MHC-I epitope presentation to CD8+ T cells is directly dependent on peptide loading and selection during antigen processing. However, the exact molecular bases underlying peptide selection and binding by MHC-I remain largely unknown. Within the peptide-loading complex, the peptide editor tapasin is key to the selection of MHC-I–bound peptides. Here, we have determined an ensemble of crystal structures of MHC-I in complex with the peptide exchange-associated dipeptide GL, as well as the tapasin-associated scoop loop, alone or in combination with candidate epitopes. These results combined with mutation analyses allow us to propose a molecular model underlying MHC-I peptide selection by tapasin. The N termini of bound peptides most probably bind first in the N-terminal and middle region of the MHC-I peptide binding cleft, upon which the peptide C termini are tested for their capacity to dislodge the tapasin scoop loop from the F pocket of the MHC-I cleft. Our results also indicate important differences in peptide selection between different MHC-I alleles.
https://doi.org/10.1073/pnas.1807656116




comments