Central residues of FSHβ (89–97) peptide are not critical for FSHR binding: Implications for peptidomimetic design
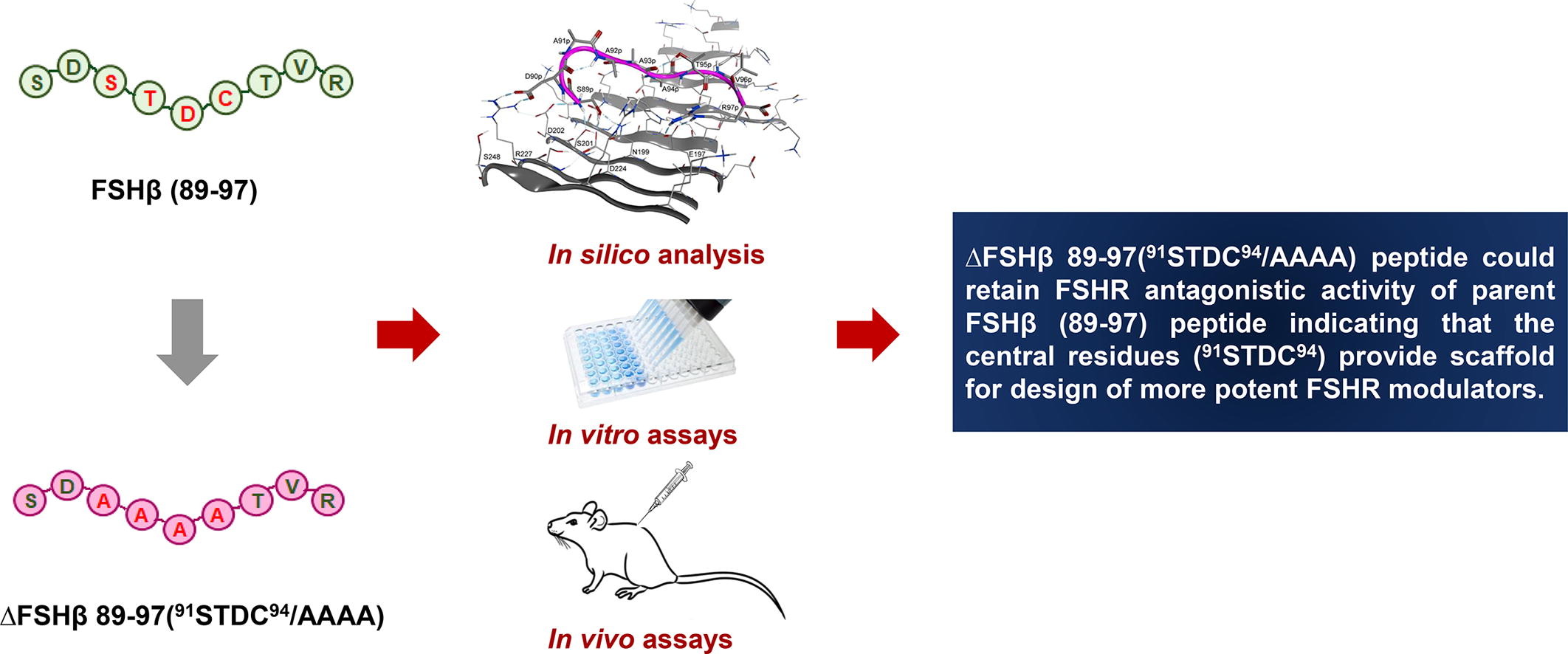
In our previous study, we had identified a 9-mer peptide (FSHβ (89–97)) derived from seat belt loop of human FSHβ and demonstrated its ability to function as FSHR antagonist in vivo. Structure analysis revealed that the four central residues 91STDC94 within this peptide may not be critical for receptor binding. In the present study, 91STDC94 residues were substituted with alanine to generate ΔFSHβ 89–97(91STDC94/AAAA) peptide. Analogous to the parent peptide, ΔFSHβ 89–97(91STDC94/AAAA) peptide inhibited binding of iodinated FSH to rat FSHR and reduced FSH-induced cAMP production. The peptide could impede granulosa cell proliferation leading to reduction in FSH-mediated ovarian weight gain in immature female rats. In these rats, peptide administration further downregulated androgen receptor and estrogen receptor-alpha expression and upregulated estrogen receptor-beta expression. The results indicate that substitution of 91STDC94 with alanine did not significantly alter FSHR antagonist activity of FSHβ (89–97) peptide implying that these residues are not critical for FSH-FSHR interaction and can be replaced with non-peptidic moieties for development of more potent peptidomimetics.





comments