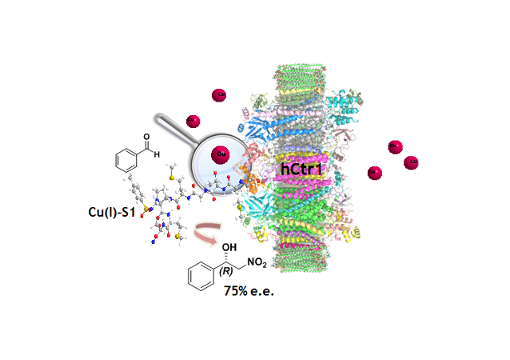
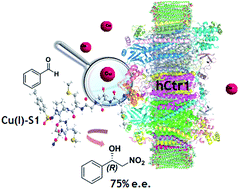
Ctr-1 Mets7 motif inspiring new peptide ligands for Cu(I)-catalyzed asymmetric Henry reactions under green conditions
DOI: 10.1039/C6RA16255J
Taking inspiration from the Ctr-1 Mets7 Cu(I) binding motif, effective hybrid catalysts have been developed for asymmetric Henry condensations under green conditions. The introduction of an unnatural dipeptide mimic allowed to increase the catalytic performance. Metal transporters’ binding domains could be exploited as a strategy for designing hybrid catalysts for homogeneous catalysis.
Hybrid catalysts have attracted the attention of several research
groups in the last few years. The development of a hybrid
catalyst involves the synergic combination of a biomacromolecular
scaffold such as a protein, and an active catalytic
moiety such as a transition metal complex. The so
obtained articial metalloenzymes have indeed shown high
selectivity in the synthesis of enantiomerically enriched
compounds in aqueous media.These successful stories have
been possible thanks to the intimate knowledge of both the
protein scaffold that hosts the metal entity and of the interaction(
s) between the metal and its chelating ligands (the first
sphere of coordination), as well as with the protein environment
(the second sphere of coordination). Nevertheless, the use of
modied protein scaffolds requires studies of site-direct
mutagenesis and molecular biology techniques. The use of
small peptides capable of binding transition metals could be
thus a much more convenient approach thanks to the easiness
and modularity of their synthesis. On the other hand, reproducing
the functional groups’ spatial arrangement of a catalytic
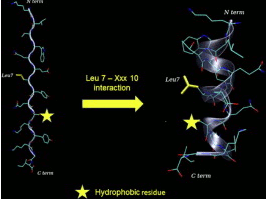
site with small peptides is still a challenge Metal transporters
are proteins, devoted to the trafficking of ions across
the membranes and contain peptide domains able to bind
metal ions. A powerful approach to design hybrid metal–
peptide catalysts could thus take advantage of the modulation
of their binding activity. The native amino acid sequence of the
binding domains could be optimized by inserting appropriate
functional amino acids. In this way it could be possible to
increase the ability to form the complex with the metal.
Furthermore, the introduction of unnatural amino acids and of
molecular scaffolds could stabilize specic conformations of
the peptide, creating the molecular architecture of a catalytic
site
comments