Morpholino-based peptide oligomers: Synthesis and DNA binding properties
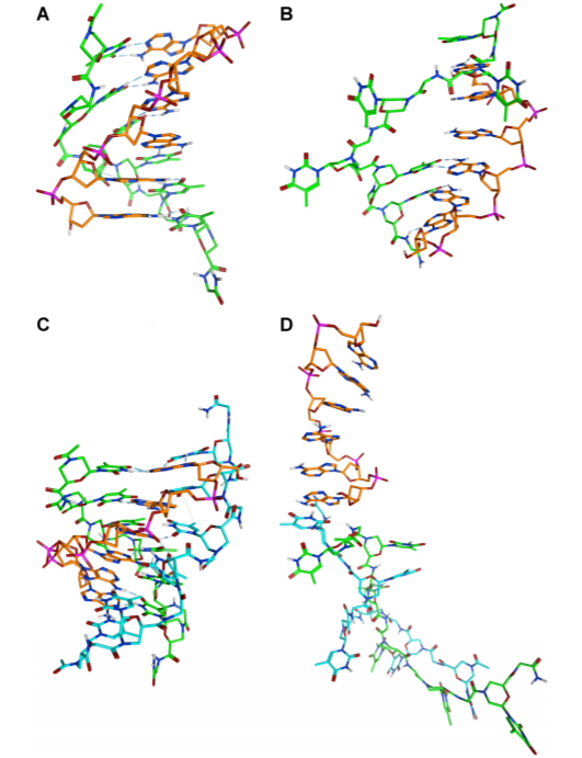
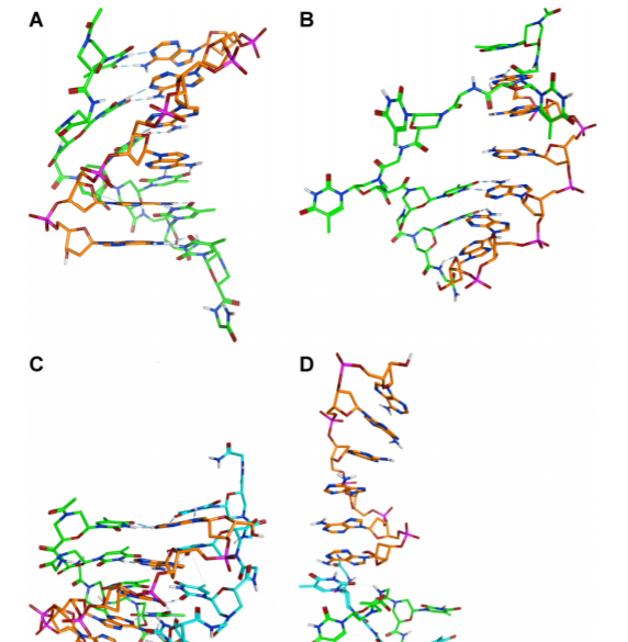
The chemical structure of oligonucleotide analogues dictates the conformation of oligonucleotide analogue oligomers, their ability to hybridize complementary DNA and RNA, their stability to degradation and their pharmacokinetic properties. In a study aimed at investigating new analogues featuring a neutral backbone, we explored the ability of oligomers containing a morpholino-peptide backbone to bind oligonucleotides. Circular Dichroism studies revealed the ability of our oligomers to interact with DNA, molecular modelling studies revealed the interaction responsible for complex stabilization.
Oligonucleotide analogues have been widely explored due to their potential application as drugs targeting nucleic acids and proteins. Modifications to the sugar moiety, to the nucleobase or to the phosphodiester backbone result in analogues with greater stability toward nucleases, increased affinity and specificity toward their target and finally improved pharmacokinetics properties ascompared to natural oligonucleotides
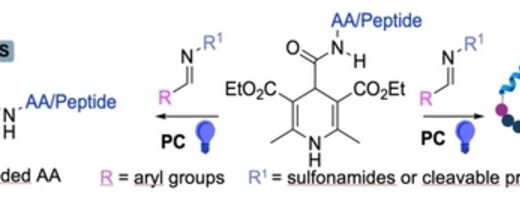




comments