Innovative On-Resin and in Solution Peptidomimetics Synthesis via Metal-Free Photocatalytic Approach
Now a days, peptidomimetics are widely studied, being useful tools in drug discovery and medicinal chemistry. The coupling between a carboxylic acid with an amine to form a peptide bond is the most common...
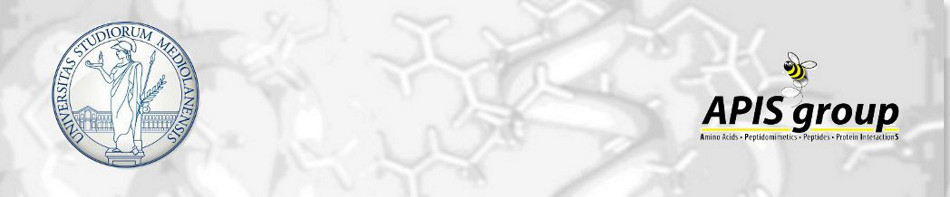
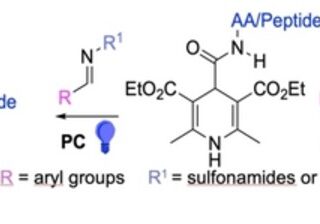
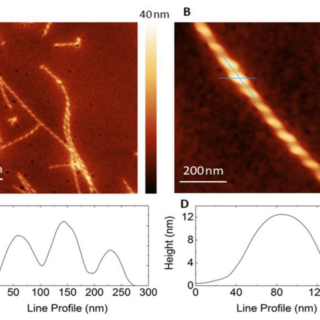

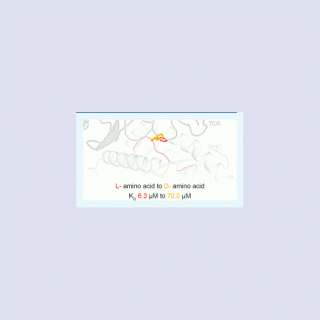
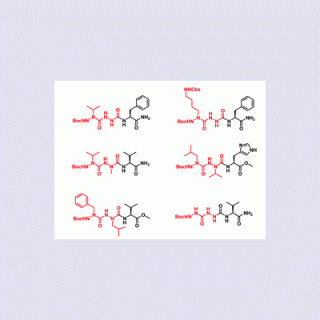
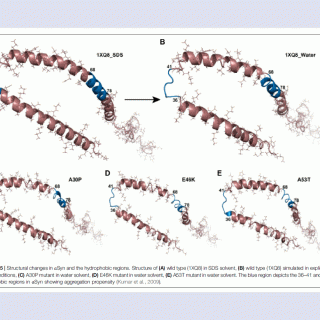
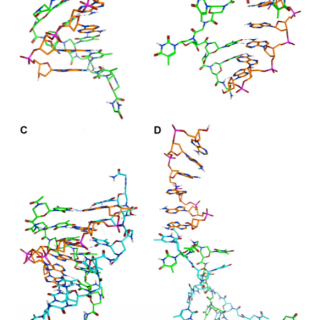
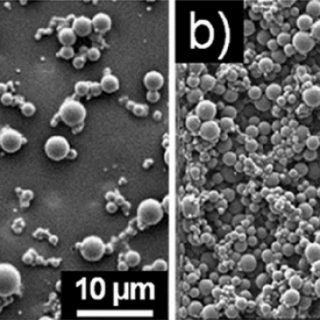
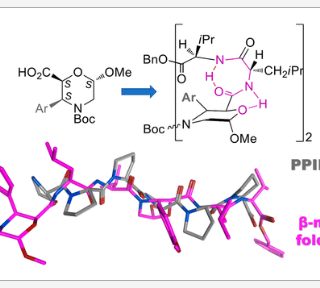




comments