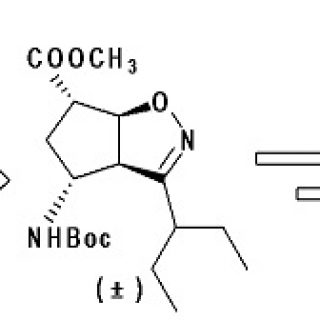
Bicyclic Pyrrolidine-Isoxazoline γ Amino Acid: A Constrained Scaffold for Stabilizing α-Turn Conformation in Isolated Peptides
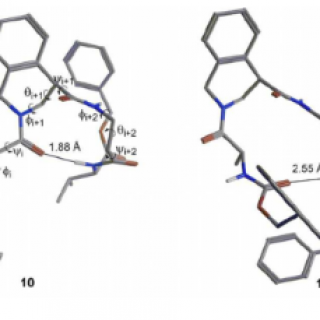
amino acids have tremendously expanded the folding possibilities of peptides and peptide mimics. While α,α-disubstituted and β-amino acids are widely studied, γ-derivatives have been less exploited. Here we report the conformational study on the...
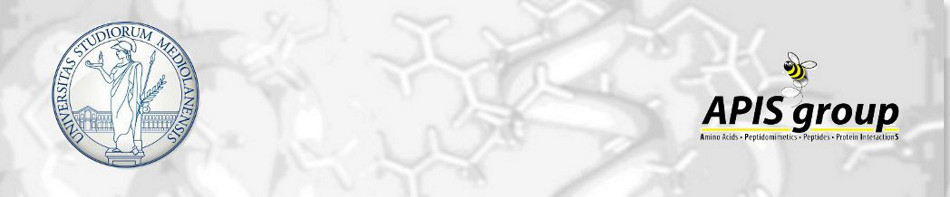
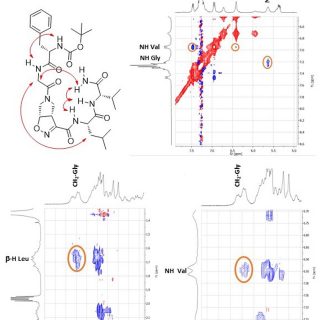
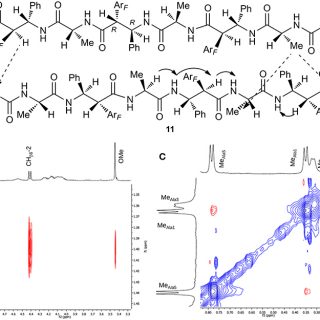
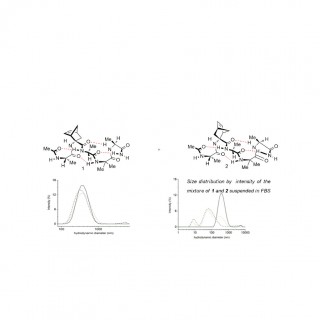
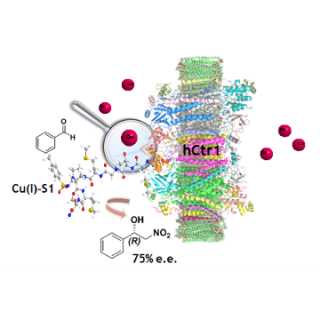
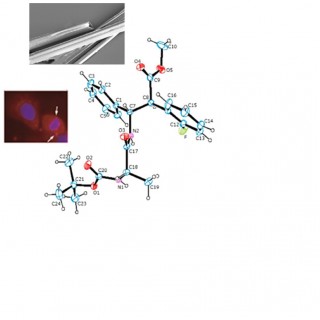




comments