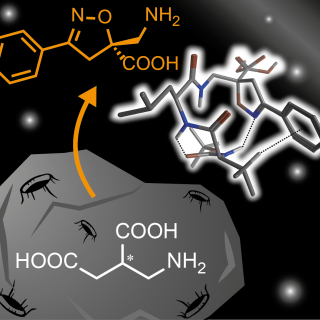
A Non-coded β2,2-Amino Acid with Isoxazoline Core Able to Stabilize Peptides Folding Through an Unprecedented Hydrogen Bond
New peptidomimetics containing a β2,2-isoxazoline amino acid, i.e. 5-(aminomethyl)-3-phenyl-4,5-dihydroisoxazole- 5-carboxylic acid (Isox-β2,2AA), were prepared and studied by NMR and theoretical calculation. Although similar amino acid derivatives have already been prepared via 1,3-dipolar cycloaddition reaction,...

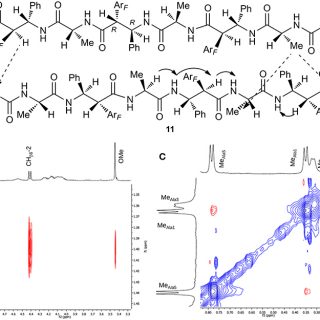
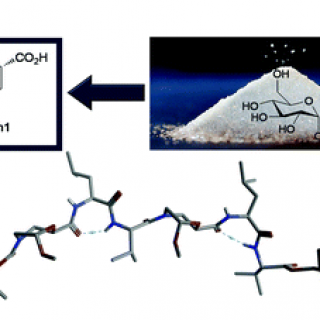




comments