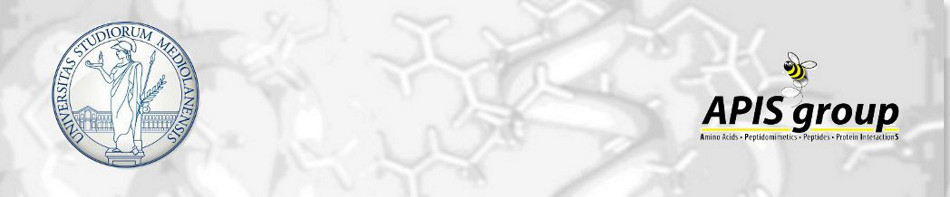
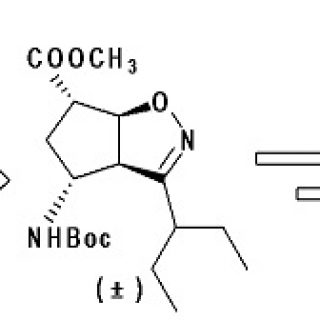
Tetrahydro‑4H‑(pyrrolo[3,4‑d]isoxazol-3-yl)methanamine: A Bicyclic Diamino Scaffold Stabilizing Parallel Turn Conformations
A tetrahydro-4H-(pyrrolo[3,4-d]isoxazol-3-yl)methanamine
scaffold was designed as a diamino derivative to stabilize parallel turn
conformations. Its synthesis took advantage of a [1,3]-dipolar cycloaddition
reaction between the nitrile oxide derived from the inexpensive enantiopure Lphenylalanine
and N-benzyl-3-pyrroline. Two diastereoisomers were formed,
whose distribution depends on the selected base. 3aR,6aS-Isomer is favored in
organic bases, which formation is driven by π-interactions. However, the above
interactions were significantly prevented using an inorganic base due to the
chaotropic effect of the cation, decreasing the amount of the above isomer.
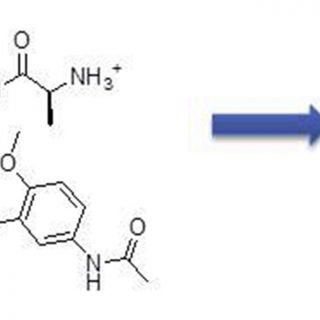
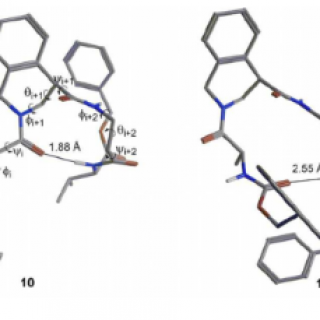
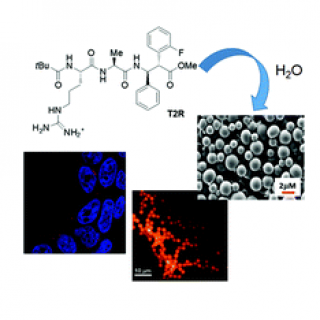
Finally, we demonstrated that this isomer is able to stabilize parallel turn
conformations when inserted in short peptide sequences.




comments