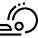Radical chemistry
Recent Advances in Enantioselective Catalytic Electrochemical Organic Transformations
F. Medici, S. Resta, S. Andolina, M. Benaglia Catalysts, 2023 13, 944 [Link]
Abstract: Different approaches can be undertaken to realise a stereoselective electrochemical synthesis. Significant contributions to enantioselective
electrochemical organic synthesis have been reported and largely reviewed in recent years. However, the development of general strategies for the electrochemical enantiocontrol
of a transformation still presents considerable challenges; in particular, relatively few contributions of highly enantioselective catalytic electrochemical reactions have been
reported to date. In this review article, the most recent examples of asymmetric electrochemical catalysis are discussed. The article is organised by the three types of
enantioselective catalysis: metal-based catalysis, organocatalysis and biocatalysis; in each section, the most significant and recent advances are presented and discussed.
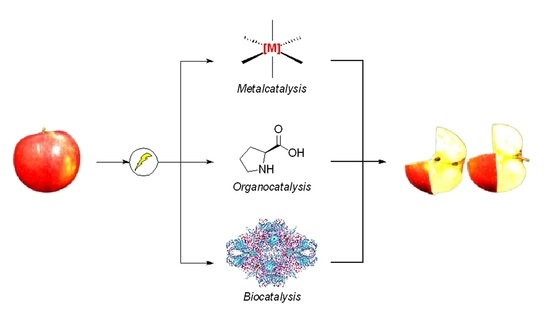
Stereoselective [2+2] Photodimerization: a Viable Strategy for the Synthesis of Enantiopure Cyclobutane Derivatives.
F. Medici, A. Puglisi, S. Rossi, L. Raimondi, M. Benaglia Org. Biomol. Chem., 2023 21, 2899-2904 [Link]
Abstract: The [2 + 2] photodimerization of cinnamic acid derivatives to afford enantiopure cyclobutanes has been investigated. The use of a chiral auxiliary
represents a convenient and straightforward method to exert enantiocontrol on the reaction. By exploiting Evans oxazolidinones, the stereoselective light-driven cyclisation
affords a functionalised cyclobutane ring with up to 99% enantiocontrol after removing the chiral auxiliary. In-flow experiments allowed us to improve further the efficiency
of the methodology, leading to high conversion and excellent enantioselectivity.
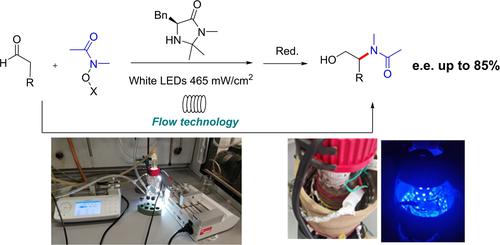
Enantioselective catalytic addition of N-acyl radicals: in batch and in flow organophotoredox α-amination of aldehydes
M. F. Boselli, N. Intini, A. Puglisi, L. Raimondi, S. Rossi, M. Benaglia Eur. J. Org. Chem, 2023 26, e2022013 [Link]
Abstract: The organophotoredox catalytic enantioselective addition of N-acyl radicals to aldehydes, to afford enantioenriched N-acyl 1,2 aminoalcohols was studied.
Under the best conditions, in batch, the product was isolated in up to 52% yield and 85% e.e., using a low cost and commercially available chiral imidazolidinone
as organocatalyst. The reaction was then studied in flow, exploring different experimental setups and photoreactors. Although modest yields were obtained, the in-flow process
afforded the product in higher productivities (up to 60 times higher) and improved space time yields (increased up to 113 times) compared to the batch reaction, with no
loss of stereoselectivity.
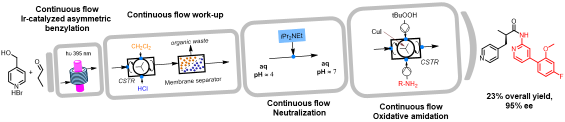
Enantioselective organophotocatalytic telescoped synthesis of a chiral privileged active pharmaceutical ingredient
F. Herbrik , M. Sanz, A. Puglisi, S. Rossi, M. Benaglia Chem. Eur. J., 2022 28 [Link]
Abstract: The continuous flow, enantioselective, organophotoredox catalytic asymmetric alkylation of aldehydes was studied, by using a homemade,
custom designed photoreactor for reactions under cryogenic conditions. Going from microfluidic conditions up to a 10 mL mesofluidic reactor, an increase
of productivity by almost 18000% compared to the batch reaction was demonstrated. Finally, for the first time, a stereoselective photoredox organocatalytic
continuous flow reaction in a fully telescoped process for an API synthesis was successfully achieved. The final process consists of 4 units of operation:
visible light-driven asymmetric catalytic benzylation under continuous flow, inline continuous work-up, neutralisation and a final oxidative amidation step
afforded the pharmaceutically active molecule in 95% e.e.
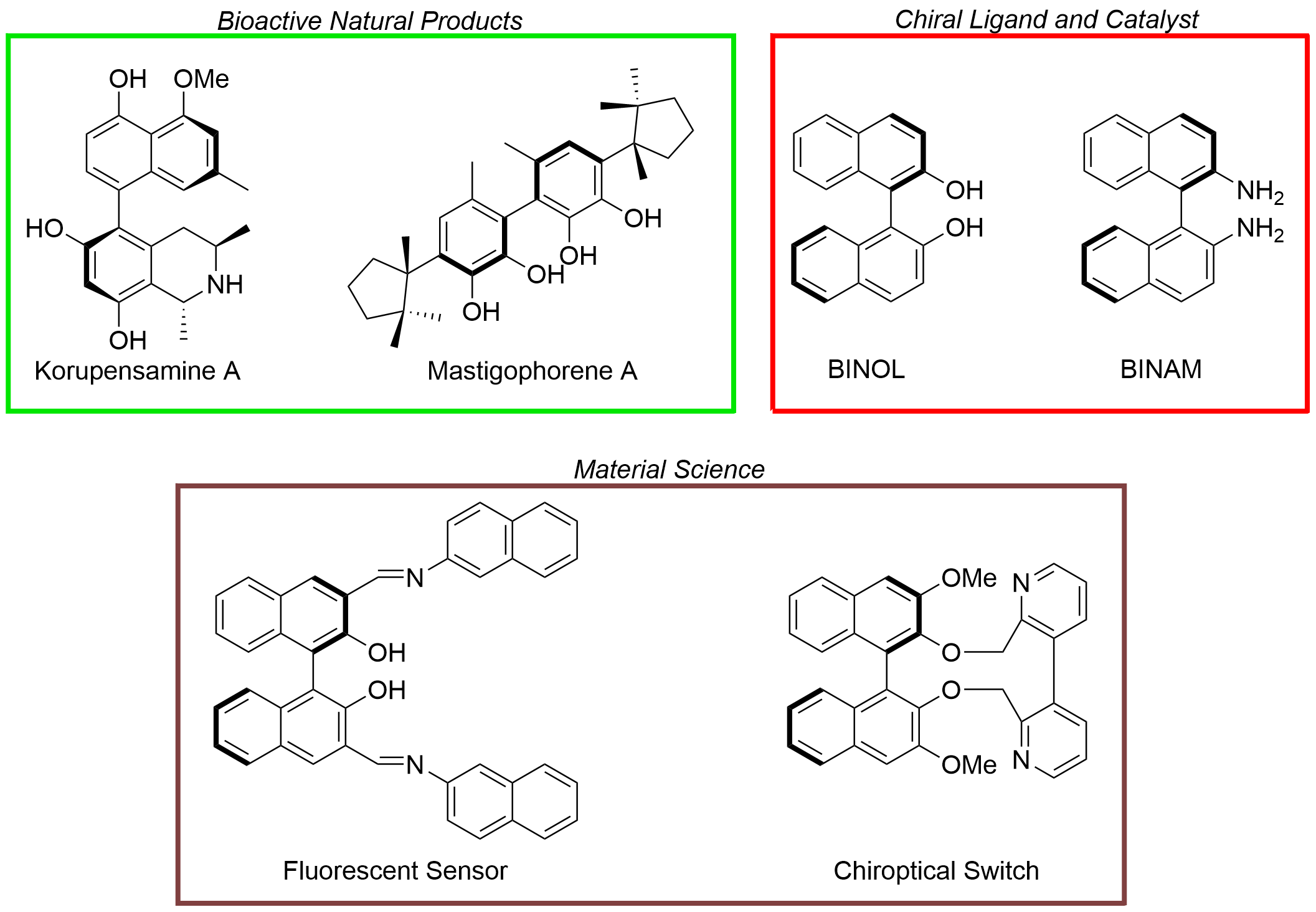
Electrochemical Organic Synthesis of Electron-Rich Biaryl Scaffolds: An Update
F. Medici, S. Resta, A. Puglisi, S. Rossi, L. Raimondi. M. Benaglia Molecules, 2021 26 22, 696828 [Link]
Abstract: Biaryl scaffolds are widely spread in biologically important natural products, in numerous therapeutic agents, but they are also considered a privileged class of ligands and (organo)catalysts; therefore, the development of efficient alternative methodologies to prepare such compounds is always attracting much attention. The present review discusses the organic electrosynthesis of biaryls starting from phenols, anilines, naphthols, and naphthylamines. The most significant examples of the works reported in the last decade are presented and classified according to the single class of molecules: after the introduction, the first three sections relate to the reactions of phenols, naphthols, and anilines, respectively; the other two sections refer to cross-coupling and miscellaneous reactions
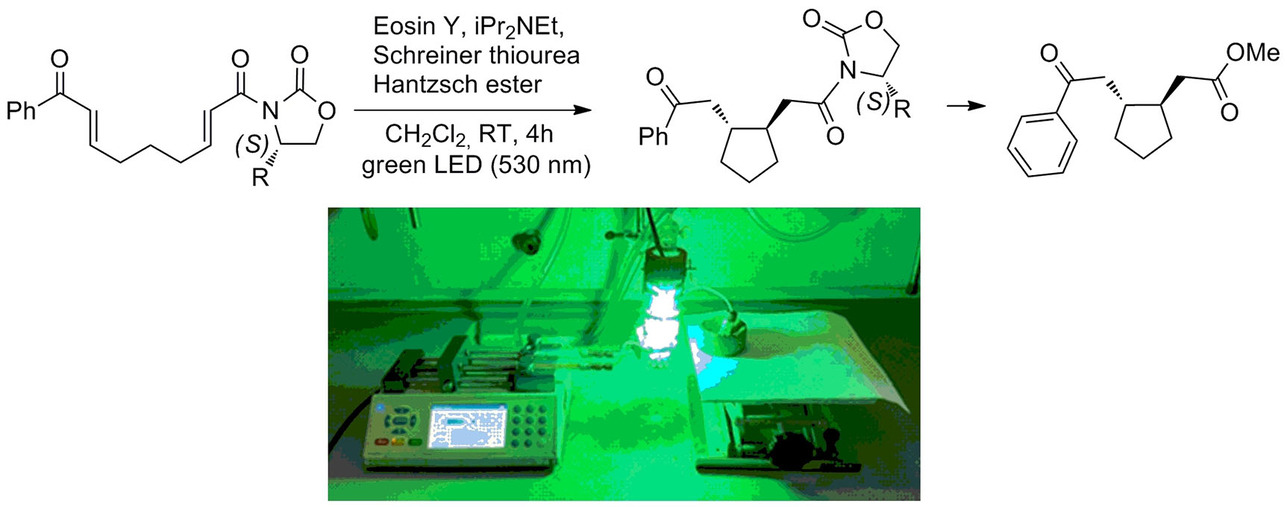
Front Cover: Stereoselective Visible-Light Catalyzed Cyclization of Bis(enones): A Viable Approach to the Synthesis of Enantiomerically Enriched Cyclopentane Rings)
F. Medici, S. Resta, P. Presenti, L. Caruso, A. Puglisi, L. Raimondi, S. Rossi, M. Benaglia Eur. J. Org. Chem., 2021 issue 32 [Link]
Abstract: The Front Cover shows how, like in a modern factory, it is possible to use light to build complex molecules in a continuous process. By exploiting chiral oxazolidinones, an efficient protocol for the in-flow synthesis of enantiomerically enriched functionalized cyclopentane rings has been developed. The stereoselective light-driven cyclization of bis(enones) in a coil photoreactor affords enantio-enriched cyclopentanes. The possibility to perform the cyclization in a 3D-printed mesoreactor was also successfully demonstrated
Stereoselective Visible-Light Catalyzed Cyclization of Bis(enones): A Viable Approach to the Synthesis of Enantiomerically Enriched Cyclopentane Rings
F. Medici, S. Resta, P. Presenti, L. Caruso, A. Puglisi, L. Raimondi, S. Rossi, M. Benaglia Eur. J. Org. Chem., 2021 4521-4524 [Link]
Abstract: An efficient protocol for the in-flow synthesis of enantiomerically enriched functionalized cyclopentane rings has been developed. By exploiting Evans’ oxazolidinones, the stereoselective light-driven cyclization of bisenones in a coil photoreactor affords, after the removal of the chiral auxiliary, an enantiomerically enriched cyclopentane. The cyclization was also successfully realized in a 3D-printed mesoreactor.