3D printing
Recent advances in the use of 3D-printed devices in organic synthesis including enantioselective catalysis [Link]
S. Rossi, A. Puglisi, L. Raimondi, M. Benaglia, Chapter in the book "Recent Advances and Perspectives in Enantioselective Catalysis" (Ed. A. Marinetti), 2021, World Scientific publisher.
Abstract: Although 3DP was initially used to evaluate prototypes, its diffusion, supported by a thriving open-source community, has reached also chemical science. In this field, 3DP technology was often combined with other enabling technologies for the realization of tissues, analytical detectors,
electrodes, and micro- and macro fluidic devices. Other applications are in separation sciences, adsorbers, airlift crystallizers, and to the realization of supplies for laboratories and basilar instrument
for chemical education purpose. Moreover, in the last two years, chemists have started to combine 3DP technology with organic compounds for the development of “3D-printed catalysts,” which have been employed in organic synthesis. In this chapter, only the very recent advances in the application of 3DP technology in organic synthesis will be discussed, with a particular focus on the implementation of the technology in batch chemistry as well as under continuous-flow conditions,
in the synthesis of drugs and pharmaceuticals and in the stereoselective synthesis of chiral molecules in the last three years (2018–2020).
3D printed catalytically active devices in organic synthesis
S. Rossi, A. Puglisi, L. Raimondi, M. Benaglia, Chimica Oggi - Chemistry today 2020, accepted
Abstract: Three-dimensional (3D) printing is a technique characterized by sequential deposition of different materials in a layer-by-layer approach for the construction of objects starting from a virtual design. The use of 3D printing technology in chemistry is becoming popular, but its potential has not been fully exploited yet. The objective of this survey is to describe the most important examples of catalytic functionalized 3D printed devices employed in synthetic transformations. For each example, the 3D printing technology used and significant results are highlighted.
Stereolithography 3D-Printed Catalytically Active Devices in Organic Synthesis
S. Rossi, A. Puglisi, L. Raimondi, M. Benaglia, Catalysts 2020, 109, 1-9. [Link]
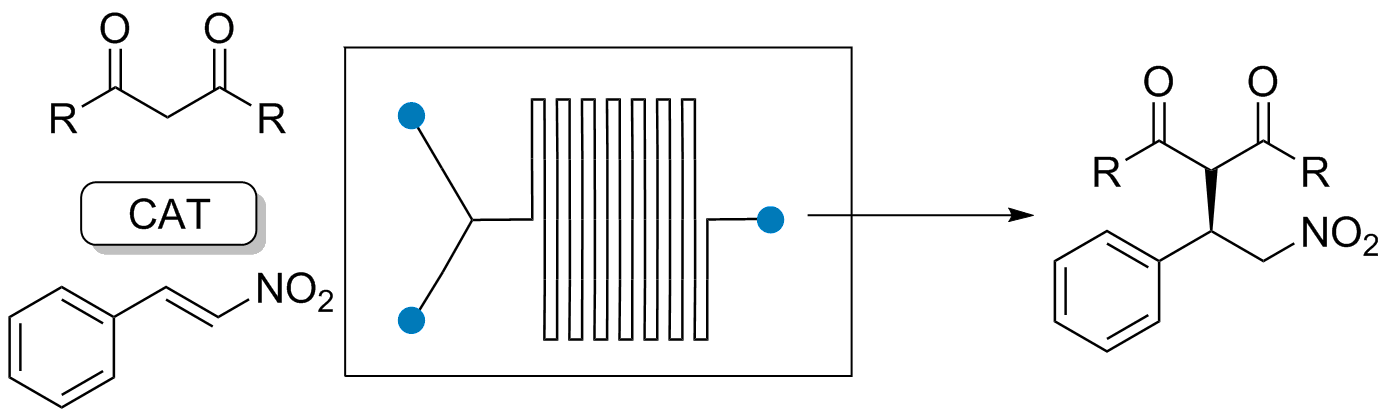
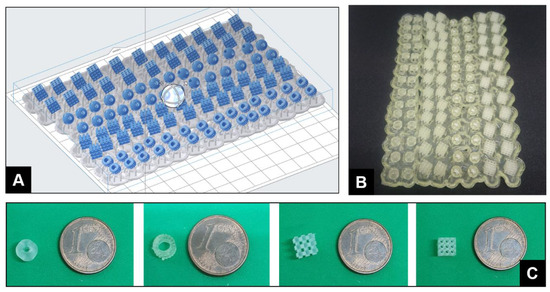
Abstract: This article describes the synthesis of stereolithography (SLA) 3D-printed catalyst-impregnated devices and their evaluation in the organocatalyzed Friedel–Crafts alkylation of N–Me–indole with trans-β-nitrostyrene. Using a low-cost SLA 3D printer and freeware design software, different devices were designed and 3D-printed using a photopolymerizable resin containing a thiourea-based organocatalyst. The architectural control offered by the 3D-printing process allows a straightforward production of devices endowed with different shapes and surface areas, with high reproducibility. The 3D-printed organocatalytic materials promoted the formation of the desired product up to a 79% yield, although with longer reaction times compared to reactions under homogeneous conditions
Additive Manufacturing Technologies: 3D printing in Organic Synthesis
S. Rossi, A. Puglisi, M. Benaglia, ChemCatChem 2018, 10, 1512-1525. [Link]
Abstract: The manufacturing of a three-dimensional product from a computer-driven digital model (3D printing) has found extensive applications in several fields. Additive manufacturing technologies offer the possibility to fabricate ad hoc tailored products on demand, at affordable prices, and have been employed to make customized and complex items for actual sale. However, despite the great progress and the countless opportunities offered by the 3D printing technology, surprisingly a relatively limited number of applications have been documented in organic chemistry. This review will focus specifically on the exploitation of additive manufacturing technologies in the synthesis of organic compounds, and, in particular, on the use of 3D-printed catalysts and 3D printed reactors, and on the fabrication and use of 3D printed flow reactors.
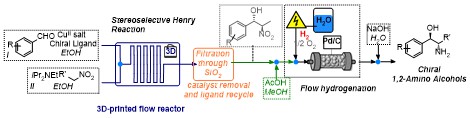
Stereoselective catalytic APIs synthesis in home-made 3D-printed mesoreactors
S. Rossi, D. Brenna, R. Porta, A. Puglisi, M. Benaglia Angew. Chem. Int. Ed., 2017, 56, 4290-4294. [Link]
Abstract: 3D-printed flow reactors were designed, fabricated from different materials (PLA, HIPS, NYLON), and used in a catalytic stereoselective Henry reaction. The use of readily prepared and tuneable 3D-printed reactors allowed for a rapid screening of devices with different sizes, shapes and channel dimensions, aimed at the identification of the best performing reactor set up. The optimized process afforded the products in high yields, moderate diastereoselectivity and up to 90% e.e.. The method was applied to the synthesis in flow of chiral 1,2-amino alcohols displaying biological activities (norephedrine, metaraminol, and methoxamine), through a two-steps, all-in-flow sequence, that involves, after the nitroaldol reaction, a continuous flow hydrogenation. To highlight the potential industrial application of this methodology, a multistep continuous synthesis of norephedrine has been realized: the product was isolated without any intermediates purification or solvent switching operation.
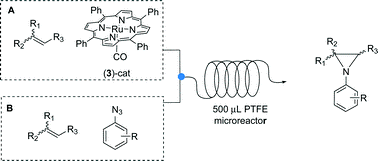
Synthesis in mesoreactors: Ru(porphyrin)CO-catalyzed aziridination of olefins under continuous flow conditions
S. Rossi, A. Puglisi, M. Benaglia, D. M. Carminati, D. Intrieri, E. Gallo Catal. Sci. Technol., 2016 [Link]
Abstract: The Ru(porphyrin)CO-catalyzed addition of aryl azides to styrenes to afford N-aryl aziridines was successfully performed for the first time in mesoreactors under continuous flow conditions. Mesofluidic technology allowed for a rapid screening of different parameters and a quick identification of the optimized reaction conditions.
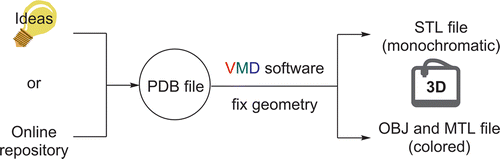
Three Dimensional (3D) Printing: A Straightforward, User-Friendly Protocol To Convert Virtual Chemical Models to Real-Life Objects
S. Rossi, M. Benaglia, D. Brenna, R. Porta, M. Orlandi J. Chem. Educ., 2015, 13, 5591-5596. [Link]
Abstract: A simple procedure to convert protein data bank files (.pdb) into a stereolithography file (.stl) using VMD software (Virtual Molecular Dynamic) is reported. This tutorial allows generating, with a very simple protocol, three-dimensional customized structures that can be printed by a low-cost 3D-printer, and used for teaching chemical education topics. With the use of the free licensed and multiplatform software, colored input geometries can be obtained by a simple-click modification procedure in order to generate .obj and .mtl files. An easy protocol to create personal .pdb files for 3D-printing technology is also reported.
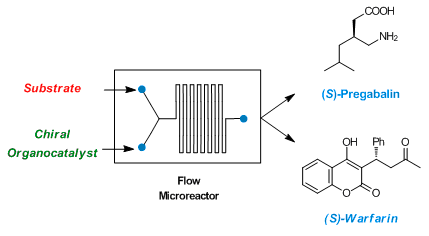
Enantioselective Organocatalysis in Microreactors: Continuous Flow Synthesis of a (S)-Pregabalin Precursor and (S)-Warfarin
R. Porta, M. Benaglia, F. Coccia, S. Rossi, A. Puglisi Symmetry, 2015, 7, 1395-1409. [Link]
Abstract: MContinuous flow processes have recently emerged as a powerful technology for performing chemical transformations since they ensure some advantages over traditional batch procedures. In this work, the use of commercially available and affordable PEEK (Polyetheretherketone) and PTFE (Polytetrafluoroethylene) HPLC (High Performance Liquid Chromatography) tubing as microreactors was exploited to perform organic reactions under continuous flow conditions, as an alternative to the commercial traditional glass microreactors. The wide availability of tubing with different sizes allowed quickly running small-scale preliminary screenings, in order to optimize the reaction parameters, and then to realize under the best experimental conditions a reaction scale up for preparative purposes. The gram production of some Active Pharmaceutical Ingredients (APIs) such as (S)-Pregabalin and (S)-Warfarin was accomplished in short reaction time with high enantioselectivity, in an experimentally very simple procedure.
Continuous-Flow Stereoselective Synthesis in Microreactors: Nucleophilic Additions to Nitrostyrenes Organocatalyzed by a Chiral Bifunctional Catalyst
S. Rossi, M. Benaglia, A. Puglisi, C.C. De Filippo, M. Maggini J. Flow Chem , 2014, 5, 17-21. [Link]
Abstract: Metal-free stereoselective additions of activated nucleophiles to β-nitrostyrenes were investigated under continuous flow conditions in microreactors, in the presence of a chiral
bifunctional catalyst. Optimization of the experimental setup gave excellent enantioselectivities (up to 85% e.e.) and higher productivities if compared to the flask syntheses.
The potential of this flow chemistry approach was demonstrated by the successful synthesis of an advanced intermediate for the preparation of the GABA-B receptor agonist Baclofen.