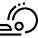Silicon Chemistry
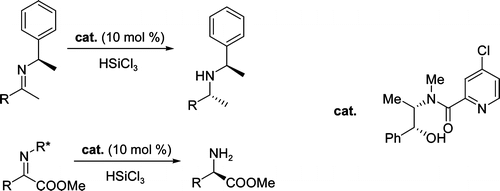
Metal-Free Deoxygenation of Chiral Nitroalkanes: An Easy Entry to α-Substituted Enantiomerically Enriched Nitriles
M. Pirola, C. Faverio, M. Orlandi, M. BenagliaChem. Eur. J. , 2021, 27 10247-10250 [Link]
Abstract: A metal-free, mild and chemodivergent transformation involving nitroalkanes was developed. Under optimized reaction conditions, in the presence of trichlorosilane and a tertiary amine, aliphatic nitroalkanes have been selectively converted into amines or nitriles. Furthermore, when chiral β-substituted nitro compounds were reacted, the stereochemical integrity of the stereocenter was maintained and β-functionalized nitriles were obtained with no loss in enantiomeric excess. The methodology was successfully applied to the synthesis of chiral β-cyano esters, α-aryl alkylnitriles, and TBS-protected cyanohydrins, including direct precursors of four APIs (Ibuprofen, Tembamide, Aegeline and Denopamine).
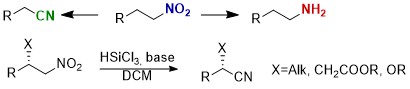
3,3’-Bithiophene-Based Chiral Bisphosphine Oxides as Organocatalysts in Silicon-Derived Lewis Acid Mediated Reactions
S. Rossi, T. Benincori, L. Raimondi, M. Benaglia Synlett, 2020, 17, 7474-7481.[Link]
Abstract: This account summarizes the development of new biheteroaromatic chiral bisphosphine oxides. 3,3′-Bithiophene-based phosphine oxides (BITIOPOs) have been successfully used as organocatalysts to promote Lewis base catalyzed, Lewis acid mediated stereoselective transformations. These highly electron-rich compounds, in combination with trichorosilyl derivatives (allyltrichlorosilane and silicon tetrachloride), generate hypervalent silicon species that act as chiral Lewis acids in highly diastereo- and enantioselective organic reactions. Several relevant examples related to these applications are discussed in detail.
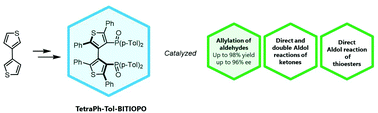
TetraPh-Tol-BITIOPO: a new atropisomeric 3,3’-bithiophene based phosphine oxide as an organocatalyst in Lewis base-catalyzed Lewis acid mediated reactions
V. M. Abbinante, M. Benaglia, S. Rossi; T. Benincori, R. Cirili, M. Pierini Org Biomol Chem, 2019, 17, 7474-7481.[Link]
Abstract: A new chiral phosphine oxide based on a 3,3’-bithiophene scaffold (TetraPh-Tol-BITIOPO) was synthesized, fully characterized and separated into antipodes through chiral HPLC. This new compound was successfully employed as an organocatalyst in Lewis base-catalyzed Lewis acid mediated reactions involving trichlorosilyl compounds. The new atropisomeric catalyst was able to promote the allylation of aldehydes with allyltrichlorosilane in up to 98% yield and up to 96% enantiomeric excess (ee), and the direct aldol reaction to afford β-hydroxy ketones and β-hydroxy thioesters, with good chemical yields and modest stereochemical efficiency. Computational studies helped to elucidate and to rationalize the stereochemical outcome of the reactions catalyzed by TetraPh-Tol-BITIOPO that was found to favour the formation of the isomer with an opposite absolute configuration in comparison with the products obtained with the previously reported 3,3’-bithiophene-based catalyst.
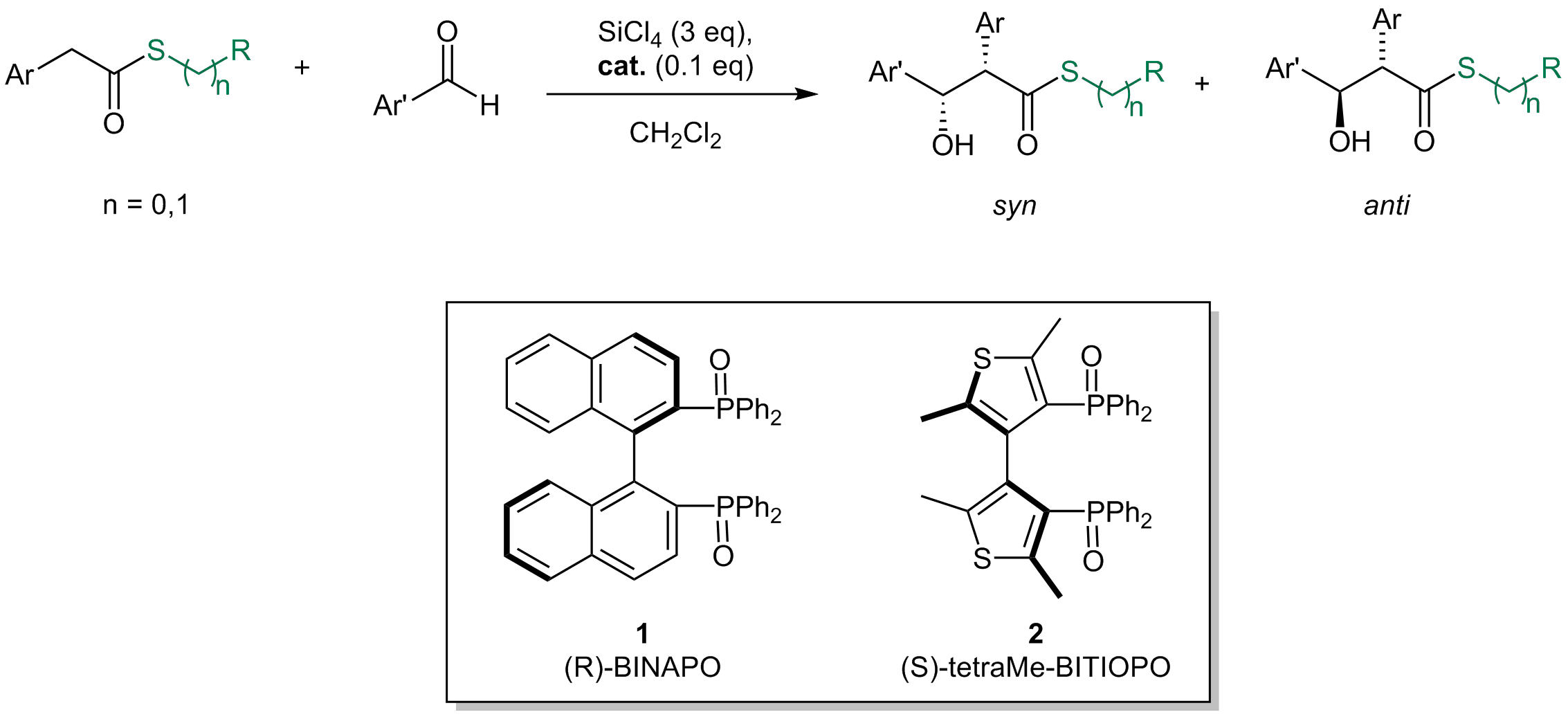
Phosphine Oxide Catalyzed, Tetrachlorosilane-Mediated Enantioselective Direct Aldol Reactions of Thioesters
S. Rossi, R. Annunziata, F. Cozzi, L. Raimondi, Synthesis, 2015, [Link]
Abstract:The stereoselective direct aldol reaction of S-phenyl thioesters and aromatic aldehydes promoted by tetrachlorosilane was realized for the first time; the proposed mechanism involves the formation of a chiral cationic hypervalent silicon species and requires the presence of catalytic amounts of a Lewis base, like a chiral phosphine oxide. The reaction affords syn ߭hydroxy thioesters as major isomers in good yields, high diastereoselectivity (up to 99:1), and up to 92% enantiomeric excess. The absolute configuration of the major isomer was established by converting the product into the corresponding ߭hydroxy ester. The scope of the reaction was also investigated by employing differently substituted thioesters in combination with different aromatic aldehydes.
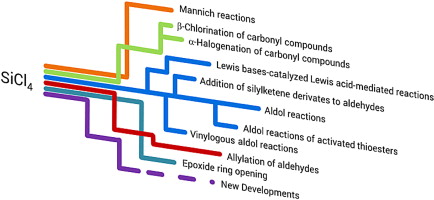
Organic reactions mediated by tetrachlorosilane.
S. Rossi, A. Genoni, M.Benaglia, tetrahedron, 2014, 70, 2065-2080 [Link]
Review:
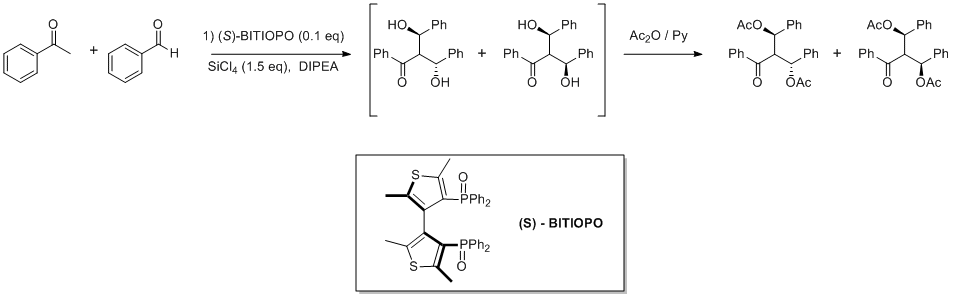
Enantiomerically Pure Bithiophene Diphosphine Oxides as Catalysts for Direct Double Aldol Reactions.
A. Genoni, M. Benaglia, S. Rossi and G. Celentano, Chirality, 2013, 25, 643-647 [Link]
Abstract: The direct aldol reaction between aryl methyl ketones with aromatic aldehydes in the presence of tetrachlorosilane and a catalytic amount of a chiral bithiophene diphosphine oxide was studied; the product of double aldol addition was isolated as diacetate in good diastereoselectivity (up to 95:5) and enantioselectivities up to 91%. The reaction with heteroaromatic aldehydes was also investigated leading to the corresponding 1,3 diols, in some cases with excellent stereoselectivities
Enantioselective reduction of ketoimines promoted by easily available (S)-proline derivatives
M. Bonsignore, M. Benaglia, L. Raimondi, M. Orlandi and G. Celentano, Beilstein J. Org. Chem., 2013, 9, 633-640. [Link]
Abstract: The behavior of readily synthesized and even commercially available (S)-proline derivatives, was studied in the trichlorosilane-mediated reduction of ketoimines. A small library of structurally and electronically modified chiral Lewis bases was considered; such compounds were shown to promote the enantioselective reduction of different substrates in good chemical yields. In the HSiCl3 addition to the model substrate N-phenylacetophenone imine, the organocatalyst of choice led to the formation of the corresponding amine with good stereoselectivity, up to 75% ee. Theoretical studies were also performed in order to elucidate the origin of the stereoselection.
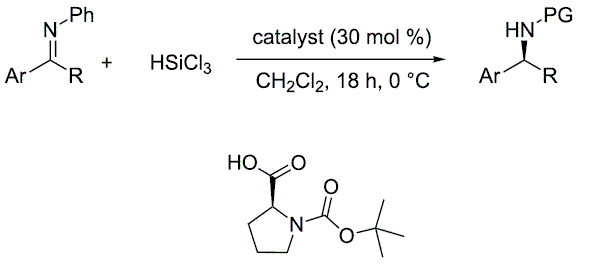
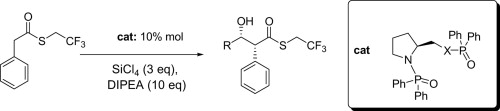
Readily available (S)-proline-derived organocatalysts for the Lewis acid-mediated Lewis base-catalyzed stereoselective aldol reactions of activated thioesters
M. Bonsignore, M. Benaglia, F. Cozzi, A. Genoni, S. Rossi, L. Raimondi, Tetrahedron, 2012, 69, 8251-8255. [Link]
Abstract: A set of enantiomerically pure Lewis bases containing different diphenyl phosphinyl oxide groups were easily synthesized in a straightforward procedure by reaction of readily available proline-based scaffolds and phosphinoyl chlorides. The newly synthesized derivatives were employed (0.1 mol equiv) as organocatalysts in the Lewis acid-mediated Lewis base-promoted direct stereoselective aldol reactions of activated thioesters with aromatic aldehydes, carried out in the presence of tetrachlorosilane and a tertiary amine. &Beta-Hydroxy thioesters were obtained in moderate to high yields, with very high syn diastereoselectivities (dr up to 98:2), and fair enantioselectivities (ee up to 51%). Theoretical studies were performed in order to elucidate the origin of the stereoselection.
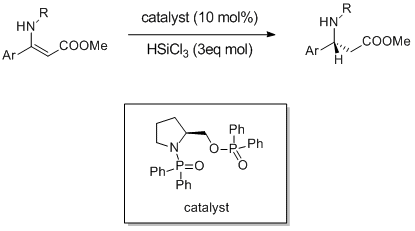
New, readily available organocatalysts for the enantioselective reduction of α-imino- and β-imino esters.
M. Bonsignore, M. Benaglia, R. Annunziata, G. Celentano, Synlett, 2011, 1085-1088. [Link]
Abstract: Novel metal-free, readily available chiral catalytic systems for the enantioselective reduction of keto imines are reported. Different β-imino esters were reduced by trichlorosilane in the presence of 10 mol% of a chiral Lewis base easily obtained in one step only from prolinol, in high yields and in up to 85% enantioselectivity; imines bearing an inexpensive and removable chiral auxiliary, were reduced with complete control of the absolute stereochemistry. The methodology was successfully extended to the stereoselective synthesis of &Beta-amino esters
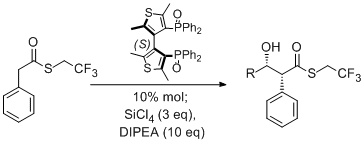
Organocatalytic stereoselective direct aldol reaction of trifluoroethyl thioesters.
S. Rossi, M. Benaglia, A. Genoni, F. Cozzi, T. Benincori, Adv. Synth. Catal., 2011, 353, 848-854. [Link]
Abstract: The first organocatalytic, stereoselective and direct aldol reaction of activated thioesters with aldehydes has been accomplished. The trichlorosilyl ketene thioacetal generated in situ by adding a tertiary amine to a trifluoroethyl thioester in the presence of tetrachlorosilane is activated by catalytic amounts of an enantiomerically pure biheteroaromatic phosphine oxide to react with different aldehydes, coordinated to as well as activated by the chiral cationic hypervalent silicon species. Starting from a variety of readily available thioesters, this Lewis acid-mediated Lewis base-catalyzed transformation allows the direct synthesis of syn-β-hydroxy thioesters in up to 95% ee.
Highly stereoselective metal-free catalytic reduction of imines: an easy entry to enantiomerically pure amines and natural and unnatural β-amino esters.
S. Guizzetti, M. Benaglia, S. Rossi, Org. Lett., 2009, 11, 2928-2931. [Link]
Abstract: A highly efficient catalytic stereoselective ketimine reduction is described. The combination of an inexpensive chiral organocatalyst, easily prepared in a single step, and of a very cheap removable chiral auxiliary allowed us to obtain enantiomerically pure amino compounds. The methodology allowed synthesis of chiral secondary and primary amines and natural and unnatural amino esters in high yields often with total control of the absolute stereochemistry.