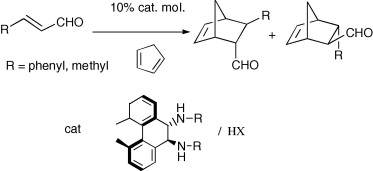
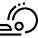H-bond mediated reactions
2-Aminoimidazolyl and 2-aminopyridyl (S)-prolinamides as versatile multifunctional organic catalysts for aldol, Michael and Diels Alder reactions.
M. Orlandi, M. Benaglia, L. Raimondi, G. Celentano, Eur. J. Org. Chem., 2013, 12, 2346-2354. [Link]
Abstract: The synthesis of multifunctional organic catalysts, easily obtained by condensation of (S)-proline with 2-amino- or 2,6-diaminopyridine, and with 2-aminoimidazole was reported. These chiral prolinamides promoted the direct aldol condensation between cyclohexanone and different aromatic aldehydes in moderate to very high enantioselectivities (up to 98% e.e.). 2-imidazolyl-derived prolinamide was succesfully employed also to promote Diels Alder and Michael reactions.
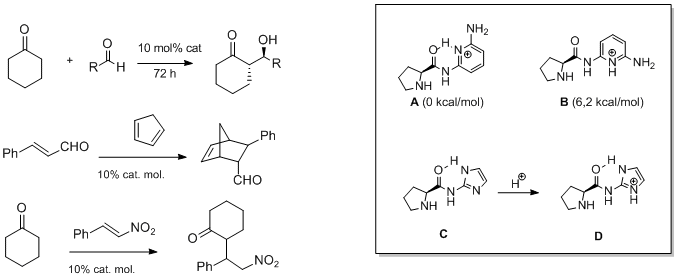
Chiral Bis-pyridinium Salts as Novel Stereoselective Catalysts for the Metal-free Diels-Alder Cycloaddition of α,β-Unsaturated Aldehydes.
A. Genoni, M. Benaglia, A. Puglisi, S. Rossi, Synthesis, 2011, 1926-1929. [Link]
Abstract: The synthesis of enantiomerically pure C2-symmetric bis-pyridinium salts was realized through a simple condensation of 2-pyridyl-carboxyaldehydes with a chiral diamine. The catalytic properties of such novel compounds were preliminarily studied and the trifluoracetate salts of chiral bis-imines of 2-picolinaldehydes derived from 1,1′-binaphthyl-2,2′-diamine were shown to catalyze the Diels-Alder reaction between cyclopentadiene and α,β-unsaturated aldehydes. Modest exo stereoselectivities and enantioselectivities of up to 55% were obtained.
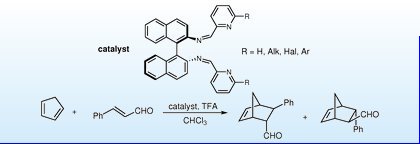
Synthesis of new chiral cyclic 1,2-diamines and their evaluation as catalysts for enantioselective Diels-Alder reactions.
C. Biaggi, M. Benaglia, S. Rossi, S. Proto, R. Annunziata, Tetrahedron Letters, 2007, 48, 8521-8525. [Link]
Abstract: The synthesis of new, enantiomerically pure cyclic 1,2-diamine structures was realized through a zinc promoted coupling of chiral bis-imines easily prepared starting from a chiral biphenyl dicarboxylic acid. The catalytic properties of such novel compounds were preliminarily studied; the trifluoroacetate salts of these chiral 1,2-diamines were shown to be able to catalyze the Diels–Alder reaction between cyclopentadiene and α,β-unsaturated aldehydes with good exo stereoselectivity and enantioselectivities up to 71%.