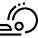Non-conventional reaction medium
Hydrogen bond-mediated organocatalytic enantioselective reduction of nitroalkenes in deep eutectic solvents
C. Faverio, M. F. Boselli, T. Ruggiero, L. Raimondi, M. Benaglia Tetrahedron Chem., 2023, 6, 100038. [Link]
Abstract: The catalytic enantioselective reduction of β,β-disubstituted nitroalkenes was performed in deep eutectic solvents, avoiding the use of volatile
organic compounds (VOCs) as reaction medium. The desired enantioenriched nitroalkanes were obtained in high yields and high enantiomeric excesses, up to 90%, by a metal-free,
hydrogen bond-mediated catalytic methodology, with a convenient experimental protocol that could be successfully applied to a gram-scale reaction.
Nitroalkene reduction in deep eutectic solvents promoted by BH3-NH3.
C. Faverio, M. Boselli, P. Camarero Gonzalez, A. Puglisi, M. Benaglia Beilstein J. Org. Chem., 2021, 17, 1041–1047. [Link]
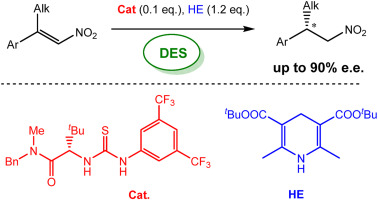
Abstract: Deep Eutectic Solvents (DESs) have gained attention as green, safe, economically and environmentally sustainable alternative to the traditional
organic solvents. Here we report the combination of an atom economy very convenient and inexpensive reagent, such as BH3NH3, in bio-based eutectic mixtures as biorenewable
solvents, in the synthesis of nitro alkanes, valuable precursors of amines. A variety of nitrostyrenes and alkyl-substituted nitroalkenes, including α- and β-
substituted nitroolefins, were chemoselectively reduced to the nitro alkanes, with an atom economy-oriented, simple and convenient experimental procedure. A reliable and
easily reproducible protocol to isolate the product without the use of any organic solvent was established and the recyclability of the deep eutectic solvent mixture was
successfully investigated.
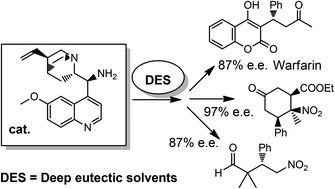
Towards the development of continuous, organocatalytic, and stereoselective reactions in deep eutectic solvents.
D. Brenna, E. Massolo, A. Puglisi, S. Rossi, G. Celentano, M. Benaglia, V. Capriati Beilstein J. Org. Chem., 2016, 12, 2620ֲ626. [Link]
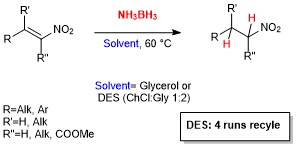
Abstract: Different deep eutectic solvent (DES) mixtures were studied as reaction media for the continuous synthesis of enantiomerically enriched products by testing different experimental set ups. L-proline-catalysed cross-aldol reactions were efficiently performed in continuo, with high yield (99%), anti-stereoselectivity, and enantioselectivity (up to 97% e.e.). Moreover, using two different DES mixtures, the diastereoselectivity of the process could be tuned, thereby leading to the formation, under different experimental conditions, to both the syn- and the anti-isomer with very high enantioselectivity. The excess of cyclohexanone was recovered and reused, and the reaction could be run and the product isolated without the use of any organic solvent by a proper choice of DES components. The dramatic influence of the reaction media on the reaction rate and stereoselectivity of the process suggests that the intimate architecture of DESs deeply influences the reactivity of different species involved in the catalytic cycle.
Stereoselective organocatalysed reactions in deep eutectic solvents: highly tunable and biorenewable reaction media for sustainable Organic synthesis.
E. Massolo, S. Palmieri, M. Benaglia, V. Capriati, F. M. Pernab Green Chem., 2015. [Link]
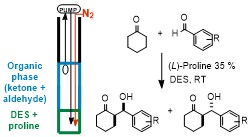
Abstract: Three distinct stereoselective reactions, catalysed by using a chiral primary amine through different activation methods, have been successfully carried out for the first time in bio-based eutectic mixtures, thereby affording functionalised molecules in very high enantioselectivity. The use of these unconventional and biorenewable reaction media also provides opportunities for facilitating the recovery and the recycling of the chiral catalyst.
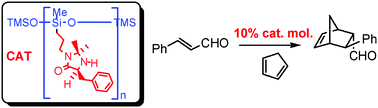
Poly(methylhydrosiloxane)-supported chiral imidazolinones: new versatile, highly efficient and recyclable organocatalysts for stereoselective Diels-Alder cycloaddition reactions.
S. Guizzetti, M. Benaglia, J. S. Siegel, Chem. Commun., 2012, 48, 3188-3190. [Link]
Abstract: Poly(methylhydrosiloxane) (PMHS) supported organic catalysts promoted the Diels-Alder reaction of dienes with a,߭unsaturated aldehydes, also in pure water, in yields and enantiomeric excesses comparable to those observed with the non-supported catalysts (up to 93% ee). Recycling of the catalysts was performed with no loss of enantioselectivity for at least five reaction cycles.
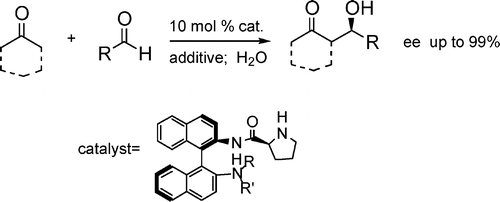
Enantioselective direct aldol reaction "on water" promoted by chiral organic catalyst.
S. Guizzetti, M. Benaglia, L. Raimondi, G. Celentano, Org. Lett., 2007, 9, 1247-1250 [Link]
Abstract: 1,1'-Binaphthyl-2,2'-diamine-based (S)-prolinamides in the presence of stearic acid were able to promote the direct aldol condensation of cyclohexanone and other ketones with different aldehydes in the presence of a massive amount of water in very good yields, high diastereoselectivity, and up to 99% ee. The behavior of both C2- and C1-symmetric catalysts in combination with different additives was investigated, and a preliminary experiment of recovering and recycling of the catalytic system was also attempted.
For other publications in this field, see here