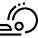Synthetic methodologies
A convenient protocol for a zinc-catalysed synthesis of electron-poor indoles
F. Medici, F. Montinari, E. Donato, L. Raimondi, M. Benaglia Tetrahedron Lett , 2023, 116, 154340 [Link]
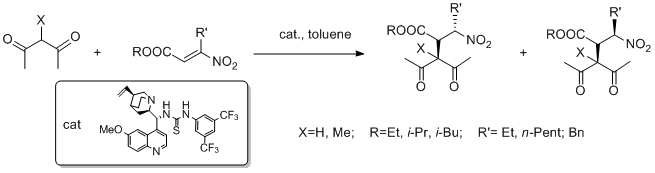
Abstract: A convenient catalytic protocol for the synthesis of electron-poor indoles was envisioned. A cyclisation reaction of electron- deficient 2-(alkynyl)-anilines, catalysed by Zn(II) salts, was successfully developed and applied to the synthesis of an API precursor. A two-step one-pot procedure starting from aniline derivatives was successfully developed. The use of a readily available, non-noble metal further enhanced the value of the protocol.
Stereoselective domino reactions in the synthesis of spiro compounds
R. Westphal, E. Venturini Filho, F. Medici, M. Benaglia, S. J. Greco Synthesis , 2022, 54, 2927–2975 [Link]
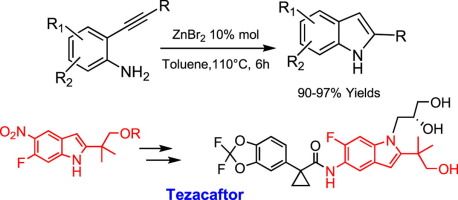
Abstract: This review summarizes the latest developments in asymmetric domino reactions, with the emphasis on the preparation of spiro compounds.
Discussions on the stereoselectivity of the transformations, the reaction mechanisms, the rationalization of the stereochemical outcome, and the applications of
domino reactions to the synthesis of biologically active molecules and natural products are included when appropriate.
1 Introduction
2 Asymmetric Domino Reactions
2.1 Domino Reactions Initiated by Michael Reactions
2.2 Domino Reactions Initiated by Mannich Reactions
2.3 Domino Reactions Initiated by Knoevenagel Reactions
2.4 Domino Reactions Initiated by Cycloaddition Reactions
2.5 Domino Reactions Initiated by Metal Insertion
2.6 Other Mechanisms
3 Conclusion
Enantioselective Povarov reactions: An update of a powerful catalytic synthetic methodology
B. C. Lemos, E. Venturini Filho, R. G. Fiorot, F. Medici, S. J. Greco, M. Benaglia Eur. J. Org. Chem. , 2022, e202101171 [Link]
Abstract: One of the best-studied routes to prepare chiral tetrahydroquinolines is known as the enantioselective Povarov reaction. In this Minireview enantioselective Povarov reactions published since 2014 are presented, according to the chiral catalyst used, either an organocatalyst or a metal complex, and discussed. When possible, special attention has been given to the rationalization of the stereochemical outcome of the reaction, discussing transition states and catalytic cycles.
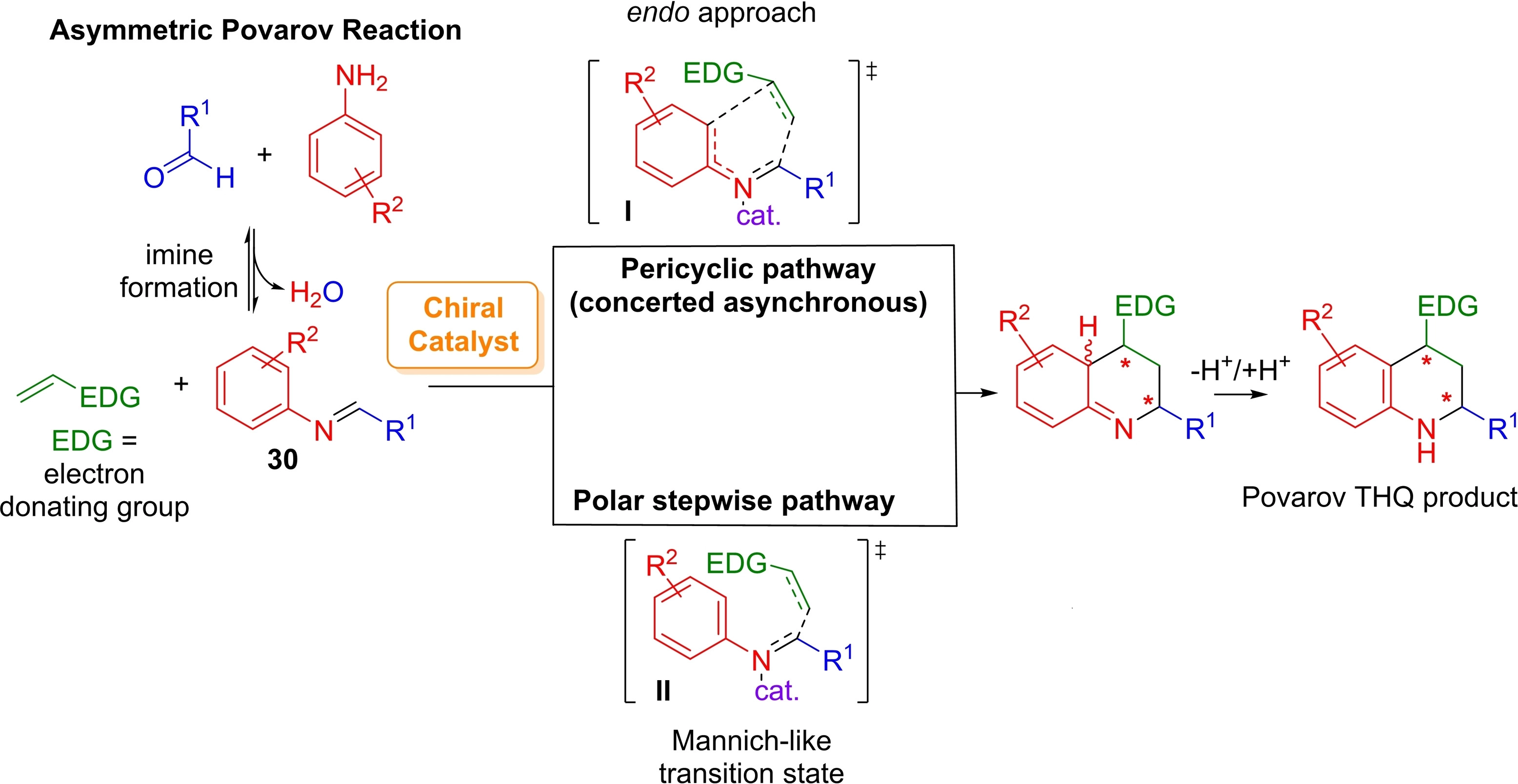
Stereoselective Synthesis of Chiral α-SCF3-β-Ketoesters Featuring a Quaternary Stereocenter
M. F. Boselli, C. Faverio, E. Massolo, L. Raimondi, A. Puglisi, M. Benaglia Symmetry , 2021, 13 92-108 [Link]
Abstract: The development of new and efficient methods, reagents, and catalysts for the introduction of a fluorine atoms or fluorinated moieties in molecular scaffolds has become a topic of paramount importance in organic synthesis. In this framework, the incorporation of the SCF3 group into organic molecule has led often to beneficial effects on the drug’s metabolic stability and bioavailability. Here we report our studies aimed to the stereoselective synthesis of chiral α-SCF3-β-ketoesters featuring a tetrasubstituted stereocenter. The use of a chiral auxiliary was crucial to synthesize enantiopure enamines that were reacted with N-trifluoromethylthio saccharin or phthalimide, to afford enantioenriched α-SCF3-tetrasubstitued β-keto esters. By using a readily available, inexpensive chiral diamine, such as trans-1,2-diaminocyclohexane, the fluorinated products could be obtained in modest to good yields, and, after the removal of the chiral auxiliary, α-substituted-α-trifluoromethylthio-β-ketoesters were isolated with up to 91% enantioselectivity.
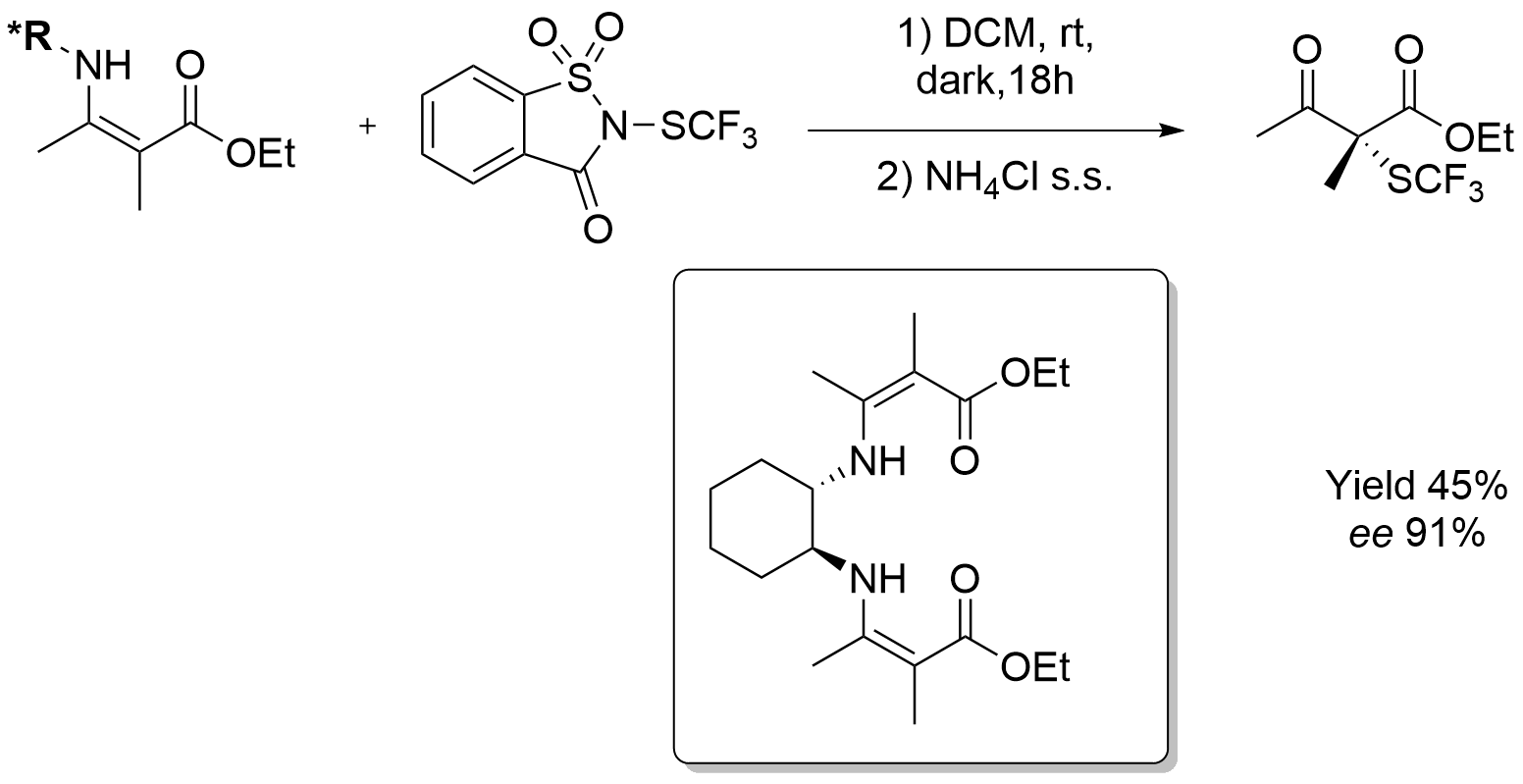
Metal-Free Deoxygenation of Chiral Nitroalkanes: An Easy Entry to α-Substituted Enantiomerically Enriched Nitriles
M. Pirola, C. Faverio, M. Orlandi, M. BenagliaChem. Eur. J. , 2021, 27 10247-10250 [Link]
Abstract: A metal-free, mild and chemodivergent transformation involving nitroalkanes was developed. Under optimized reaction conditions, in the presence of trichlorosilane and a tertiary amine, aliphatic nitroalkanes have been selectively converted into amines or nitriles. Furthermore, when chiral β-substituted nitro compounds were reacted, the stereochemical integrity of the stereocenter was maintained and β-functionalized nitriles were obtained with no loss in enantiomeric excess. The methodology was successfully applied to the synthesis of chiral β-cyano esters, α-aryl alkylnitriles, and TBS-protected cyanohydrins, including direct precursors of four APIs (Ibuprofen, Tembamide, Aegeline and Denopamine).
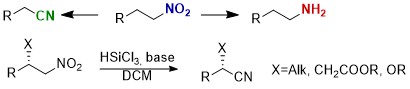
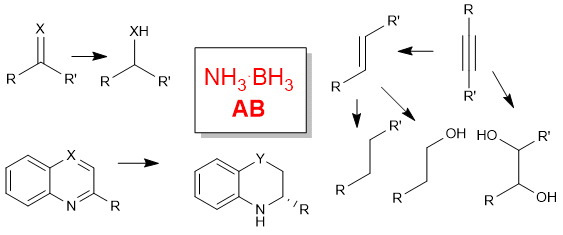
Ammonia-borane mediated reduction of nitroalkenes
C. Faverio, M. F. Boselli, L. Raimondi, M. Benaglia SynOpen, 2020, 4 116-122 [Link]
Abstract: Ammonia borane (AB) has been successfully employed in the reduction of nitroalkenes. A variety of nitrostyrenes and alkyl-substituted nitroalkenes were chemoselectively reduced to the corresponding nitroalkanes, in short reaction time, with an atom-economic, simple experimental procedure that also works with α- and β-substituted nitroolefins.
Ammonia borane as a reducing agent in organic synthesis
C. Faverio, M.F. Boselli, F. Medici, M. Benaglia Org. Biomol. Chem , 2020, accepted [Link]
Abstract: Ammonia borane NH3.BH3 is considered a promising material for hydrogen storage and release, and is attracting an increasing attention as relatively inexpensive, atom economy-convenient and viable reagent for developing new green synthetic transformations. The present review offers a wide overview on the use of AB in the reduction of organic compounds, and highlights the versatility of this reagent, due to the possibility to modulate its activity employing different strategies, that include the use of transition metals, p-block species, organocatalysts and FLP systems
 >
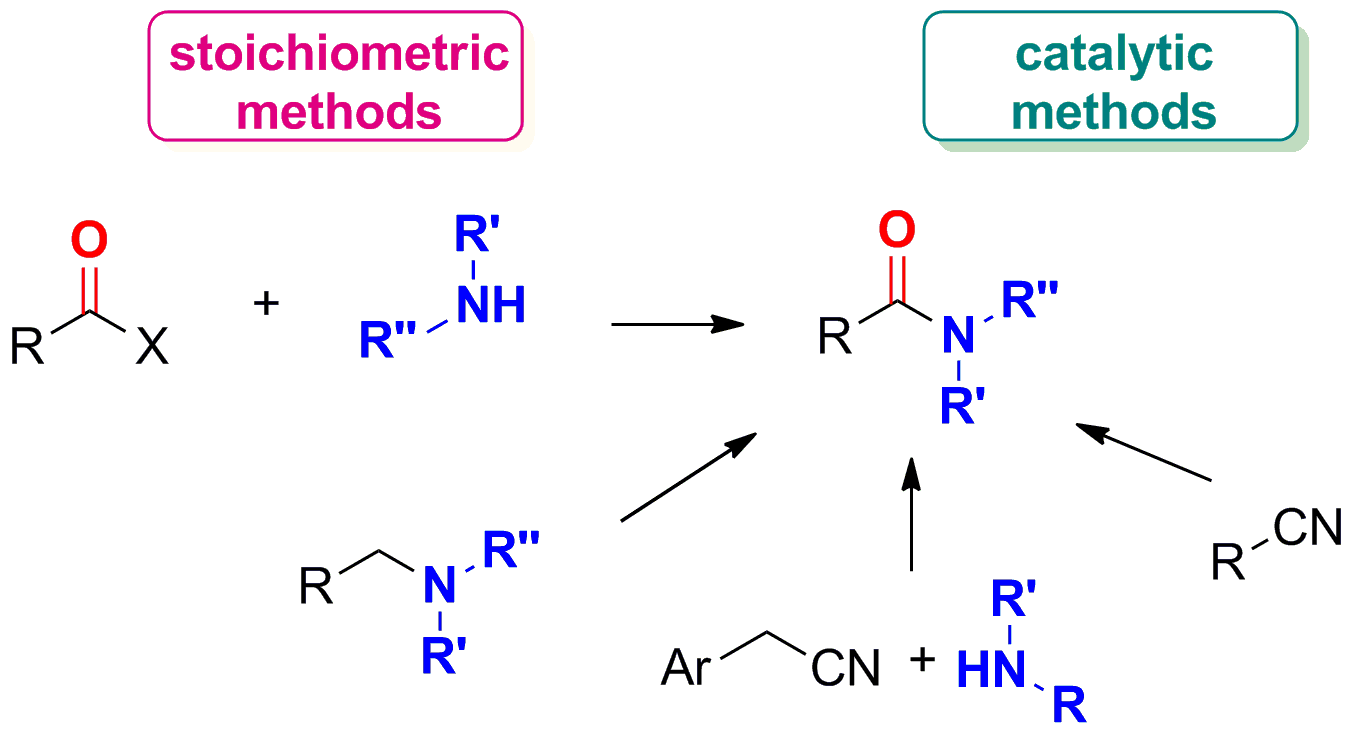
>Amide bond formation strategies: latest advances on a dateless transformation
E. Massolo, M. Pirola, M. Benaglia Eur. J. Org. Chem, 2020, accepted [Link]
Abstract:T he synthesis of amides remains one of the most important transformations and it is one of the more frequently performed reactions. In the pharmaceutical industry, building of the amide group is pivotal and among the more important transformations in the design of the synthetic plan. This review presents an overview of only very recent contributions, published in the last three years, to highlight the latest progresses in this “dateless” reaction, with a special focus on metal-free methodologies. New, more efficient and/or greener stoichiometric methods as well as catalytic strategies have been discussed, either for the “classical” coupling approach between an amine and a carboxylic acid (or its activated equivalent), and for more innovative approaches, mainly involving oxidation procedures to generate amides starting from amines
 >
>Formal α-trifluoromethylthiolation of carboxylic acid derivatives via N-acyl pyrazoles
F. Franco, S. Mennino, A. Lattanzi, M. Benaglia Chem Comm , 2020, accepted [Link]
Abstract: We disclosed a one-pot two-step protocol for the first direct, base-catalyzed α-trifluoromethylthiolation of carboxylic acid derivatives by using readily available N-acyl pyrazoles, N-(trifluoromethylthio)-phthalimide and a nucleophile such as amines, alcohols and water. Straightforward elaboration of the products to alcohols and triflones expands further the synthetic utility of the process.
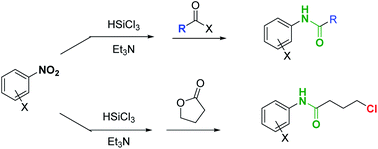
A one pot protocol to convert nitro-arenes into N-aryl amides
E. Massolo, M. Pirola, A. Puglisi, L. S. Rossi, M. Benaglia RSC Advances, 2020, 10, 4040-4044 [Link]
Abstract: A two-step one pot, experimentally simple protocol, based on readily available and inexpensive reagents allowed to convert nitro-arenes directly to N-aryl amides. A metal-free reduction of the nitro group, mediated by trichlorosilane, followed by the addition of an anhydride afforded the corresponding N-aryl carboxyamide, that was isolated after a simple aqueous work up in good-excellent yields. When the methodology was applied to the reaction with γ-butyrolactone, the desired N-aryl butanamide derivative was obtained, featuring a chlorine atom at the γ-position, a functionalized handle that can be used for further synthetic manipulation of the reaction product. Such intermediate has been already employed as key advanced precursor of pharmaceutically active compounds.
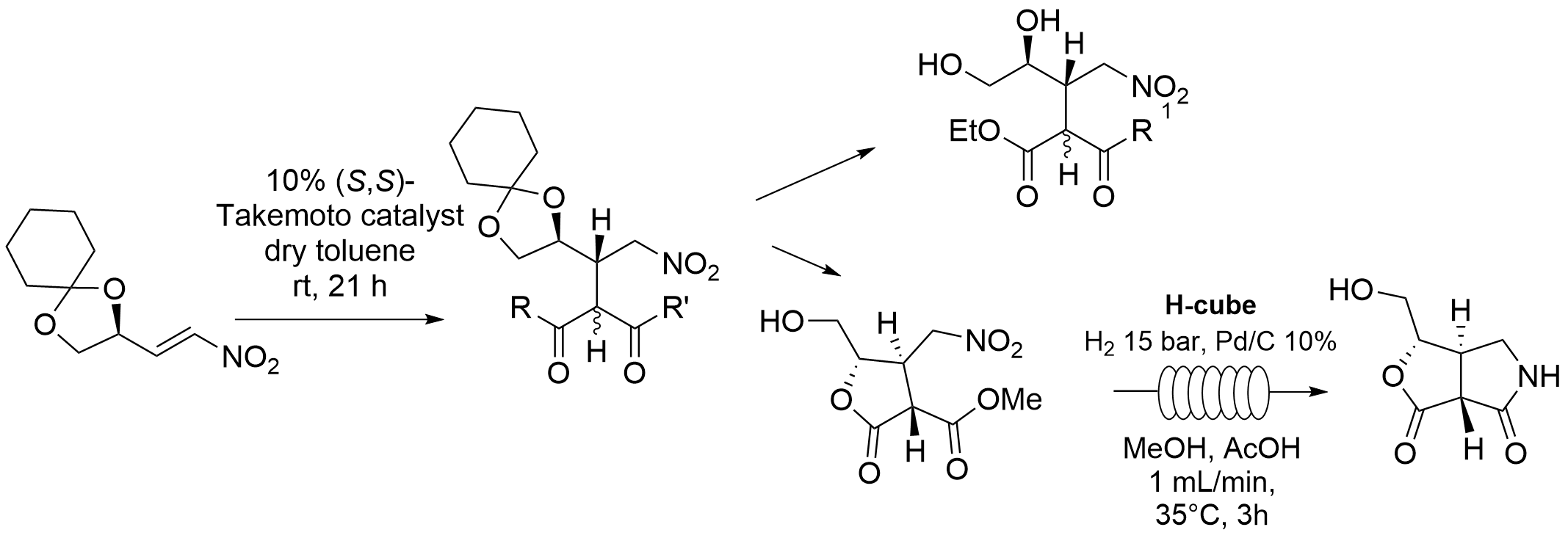
Organocatalytic Michael addition to (D)-mannitol-derived enantiopure nitroalkenes: a valuable strategy for the synthesis of densely functionalized chiral molecules.[Link]
L. Caruso, A. Puglisi, E. Gillon, M. Benaglia, molecules, 2019, 24, 4588-4597
Abstract: (D)-mannitol was selected as valuable platform, directly available from the chiral pool, to build highly functionalized molecules. Starting from (R)-2,3-O-cyclohexylidene glyceraldehyde, easily prepared on large scale from D-mannitol, an enantiopure chiral nitro alkene was prepared, by reaction with nitromethane, and its reactivity studied. Organocatalytic Michael addition of dimethyl malonate, β-keto esters and other nucleophiles on the nitro alkene afforded with high stereoselectivity densely functionalised chiral molecules, which were further synthetically elaborated, leading to five-membered lactones and bicyclic lactams. Preliminary studies showed that the metal-free catalytic reaction on the chiral nitro alkene can be performed also under continuous flow conditions, thus opening the way to the use of (micro)meso fluidic systems for the preparation of enantiomerically pure organic molecules from the chiral pool.
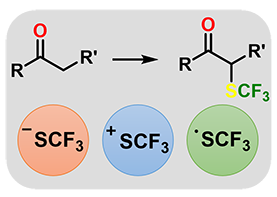
Synthesis of alpha-trifluoromethylthio carbonyl compounds: a survey of the methods for the direct introduction of the SCF3 group on to organic molecules.
S. Rossi, A. Puglisi, L. Raimondi, M. Benaglia, ChemCatChem, 2018, 10, 2717-2733. [Link]
Abstract: The role of fluorine atoms in drug discovery has become of fundamental importance, due to their ability to confer unprecedented therapeutic profiles on a molecule. In this framework, the trifluoromethylthio group (SCF3) is attracting an increasing attention in pharmaceutical, agrochemical and material chemistry and it is commonly used to modulate lipophilicity, bioavailability and metabolic stability of newly designed molecules. Actually, several drugs whose biological activity is strictly related to the presence of a SCF3 residue in the molecular scaffold are already on the market. Despite trifluoromethylthiolated carbonyl derivatives present a high potential of application in medicinal chemistry, synthetic approaches to α-SCF3-substituted carbonyl compounds are still limited, and catalytic strategies to access optically active functionalized carbonyl compounds are almost unexplored. The present review will discuss the use of radical, nucleophilic and electrophilic trifluoromethylthiolating reagents, to synthesize decorated trifluoromethylthio carbonyl derivatives, with a particular attention on catalytic methodologies and stereoselective methods affording enantiomerically enriched molecules.
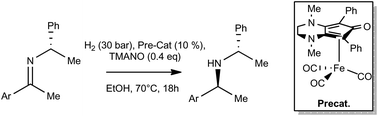
Iron catalyzed diastereoselective hydrogenation of chiral imines.
D. Brenna, S. Rossi, F. Cozzi, M. Benaglia Org. Biomol. Chem, 2017, 15, 5685-5688. [Link]
Abstract:Cyclopentadienone-based iron complexes were used for the first time to successfully catalyze the diastereoselective hydrogenation of enantiopure imines. Chiral amines, including valuable biologically active products, were obtained often as enantiomerically pure compounds. Computational studies helped to elucidate the chemical and stereochemical aspects of the iron-catalyzed reaction.
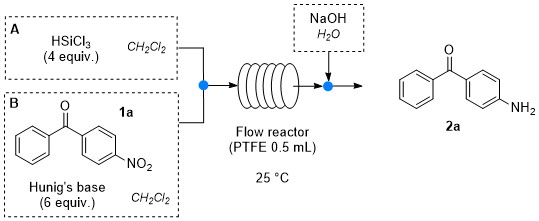
Continuous-flow synthesis of primary amines: Metal-free reduction of aliphatic and aromatic nitro derivatives with trichlorosilane.
R. Porta, A. Puglisi, G. Colombo, S. Rossi, M. Benaglia Beilstein J. Org. Chem, 2016, 12, 2614ֲ619. [Link]
Abstract:The metal-free reduction of nitro compounds to amines mediated by trichlorosilane was successfully performed for the first time under continuous flow conditions. Aromatic as well as aliphatic nitro derivatives were converted to the corresponding primary amines in high yields and very short reaction times with no need for purification. The methodology was also extended to the synthesis of two synthetically relevant intermediates (precursors of Baclofen and Boscalid).
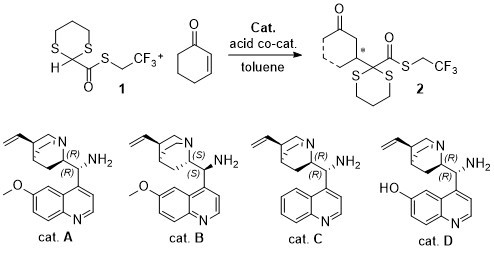
2-Carboxythioester-1,3-dithiane: a Functionalized Masked Carbonyl Nucleophile for the Organocatalytic Enantioselective Michael Addition to Enones
E. Massolo, D. Brenna, F. Cozzi, L. Raimondi, N. Gaggero M. Benaglia Synlett, 2016, accepted [Link]
Abstract:A S-(2,2,2-trifluoroethyl) 1,3-dithiane-2-carbothioate has been successfully employed as acyl anion synthon in the organocatalytic enantioselective addition to enones promoted by quinine- and quinidine-derived tertiary/primary diamines. By proper selection of a co-catalyst and by optimization of the reaction parameters, convenient experimental conditions were found, that allowed to obtain the highly functionalized products in up to 90% yield and 98% ee in short reaction times. These compounds, featuring selectively removable functionalities, proved to be versatile synthetic intermediates, which could be transformed into different derivatives without any erosion of the stereochemical integrity of the molecules
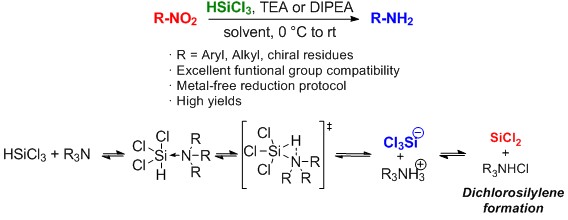
HSiCl3-mediated Reduction of Nitro-derivatives to Amines: Is Tertiary amine-stabilized SiCl2 the Actual Reducing Species?
M. Orlandi, M. Benaglia, F. Tosi, R. Annunziata, F. Cozzi J Org chem, 2016, [Link]
Abstract: The mechanism of a recently reported, highly chemoselective metal-free protocol of wide general applicability for the reduction of aromatic and aliphatic nitro-derivatives to amines has been investigated. The reaction is supposed to occur through the generation of a Si(II) reducing species; quantum mechanical calculations, spectroscopic and experimental data strongly suggest the tertiary amine-stabilized dichlorosilylene to be the most probable reducing agent.
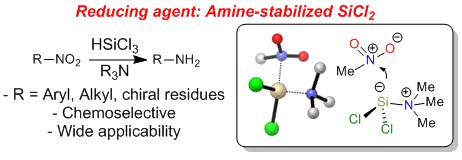
Metal-Free Reduction of Aromatic and Aliphatic Nitro Compounds to Amines: A HSiCl3-Mediated Reaction of Wide General Applicability
M. Orlandi, F. Tosi, M. Bonsignore, and M. Benaglia Org Lett, 2015, 17, 3941-3943 [Link]
Abstract: A new, mild, metal-free, HSiCl3-mediated reduction of both aromatic and aliphatic nitro groups to amines that is of wide general applicability, tolerant of many functional groups, and respectful of the stereochemical integrity of stereocenters is reported.
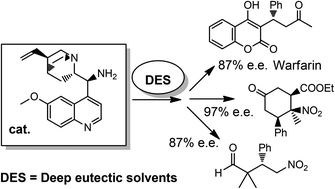
Stereoselective organocatalysed reactions in deep eutectic solvents: highly tunable and biorenewable reaction media for sustainable organic synthesis
E. Massolo, S. Palmieri, M. Benaglia, V. Capriati, F. M. Pernab Green. Chem., 2015, in press [Link]
Abstract: Three distinct stereoselective reactions, catalysed by using a chiral primary amine through different activation methods, have been successfully carried out for the first time in bio-based eutectic mixtures, thereby affording functionalised molecules in very high enantioselectivity. The use of these unconventional and biorenewable reaction media also provides opportunities for facilitating the recovery and the recycling of the chiral catalyst.
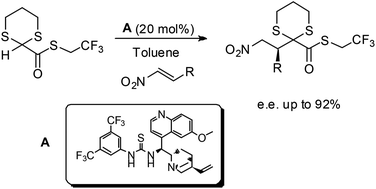
Stereoselective reaction of 2-carboxythioesters-1,3-dithiane with nitroalkenes: an organocatalytic strategy for the asymmetric addition of a glyoxylate anion equivalent.
E. Massolo, M. Benaglia, A. Genoni, R. Annunziata Org. Biomol. Chem., 2015, 13, 5591-5596 [Link]
Abstract: An efficient organocatalytic methodology has been developed to perform the stereoselective addition of 2-carboxythioesters-1,3-dithiane to nitroalkenes. Under mild reaction conditions γ-nitro-β-aryl-α-keto esters in up to 92% e.e. were obtained, realizing a formal catalytic stereoselective conjugate addition of the glyoxylate anion synthon. The reaction products are versatile starting materials for further synthetic transformations; for example, the simultaneous reduction of the nitro group and removal of the dithiane ring was accomplished, allowing the preparation of the GABAB receptor agonist baclofen.
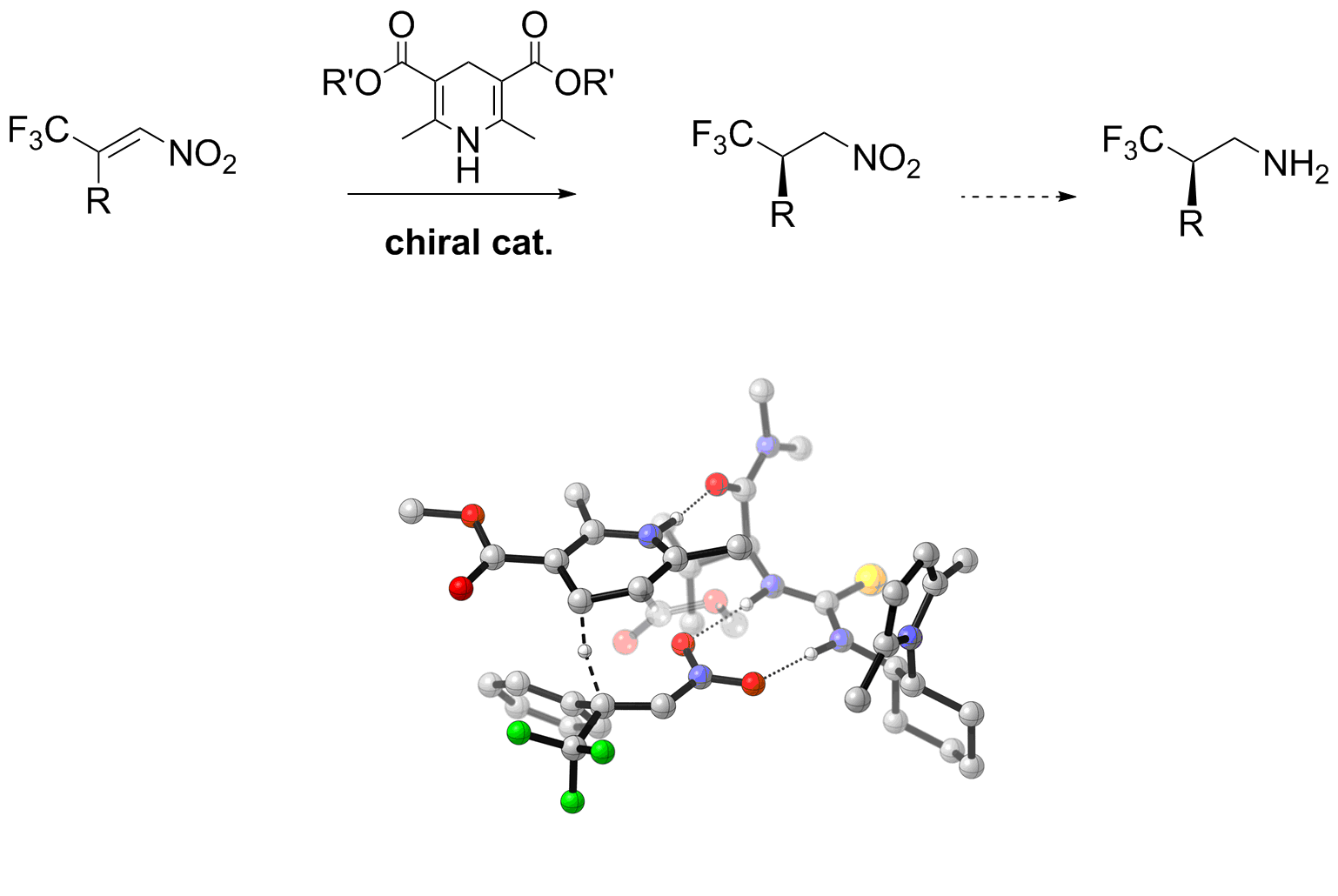
Enantioselective organocatalytic reduction of β-trifluoromethyl nitroalkenes: an efficient strategy for the synthesis of chiral β-trifluoromethyl amines.
E. Massolo, M. Benaglia, M. Orlandi, S. Rossi, G. Celentano Chemistry, Eur. J., 2015, accepted [Link]
Abstract: An efficient organocatalytic stereoselective reduction of β--trifluoromethyl-substituted nitroalkenes, mediated by 3,5-dicarboxylic esters-dihydro pyridines (Hantzsch ester type), has been
successfully developed. A multifunctional thiourea-based (S)-valine derivative was found to be the catalyst of choice, promoting the reaction in up to 97% ee. The methodology has been applied
to a wide variety of substrates, leading to the formation of differently substituted precursors of enantiomerically enriched β-trifluoromethyl amines. The mechanism of the reaction and the
mode of action of the metal-free catalytic species were computationally investigated; on the basis of DFT transition states (TS) analysis, a model of stereoselection was also proposed.
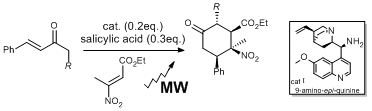
Synthesis of highly decorated chiral 2-nitro-cyclohexane carboxylic esters through microwave-assisted organocatalyzed cascade reactions.
E. Massolo, M. Benaglia, D. Parravicini, D. Brenna, R. Annunziata Tetrahedron Letters , 2014, 55, 6639-6642 [Link]
Abstract: Starting from (E)-β-substituted-β-nitroacrylates and α,β-unsaturated ketones, a stereoselective organocatalyzed one-pot methodology allowed to synthesize highly decorated chiral 2-nitro-cyclohexane carboxylic esters. The reaction is promoted by Cinchona alkaloids-derived primary amines in the presence of an acidic co-catalyst and affords two diastereoisomers, in good yields and high enantiomeric excess (often higher than 90% e.e.). By replacing conventional heating with microwave irradiation, cleaner reactions in shortened times (from 48 hours to 30 minutes) were obtained, with improved d.r. (80:20) and high e.e. (up to 94%). The application of microwave technology to this organocatalytic methodology allowed also employing C1 substituted enones, leading to cyclohexanones with four contiguous stereocenters in two isomers only, and up to 99% enantioselectivity.
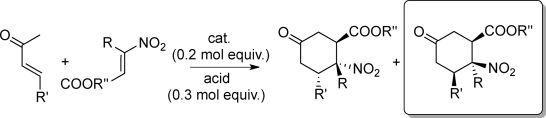
Stereoselective Synthesis of Functionalized Chiral 2-Nitrocyclohexanecarboxylic Esters via Catalytic Dienamine Addition to β-Substituted β-Nitroacrylates
E. Massolo, M. Benaglia, R. Annunziata, A. Palmieri, G. Celentano, A. Forni, Adv. Synth. Catal., 2014, 356, 493-500 [Link]
Abstract: A metal-free stereoselective catalytic addition of in situ generated dienamine to β-nitroacrylates has been developed. Starting from simple α,β-unsaturated ketones, highly functionalized chiral β-nitrocyclohexanecarboxylic esters were obtained in a single step, in good yields and up to 98% ee. By an unprecedented mechanistic pathway, starting from the synthetically readily available E-nitroacrylate, the present methodology allowed us to obtain as major product the isomer bearing a cis relative disposition between the nitro and the ester groups, which is not accessible via a classical Diels-Alder reaction.
Stereoselective addition reactions to β-nitroacrylates catalyzed by chiral metal-free bifunctional catalysts.
E. Massolo, M. Benaglia, A. Palmieri, and G. Celentano, Asian J. Org. Chem, 2014, 3, 416-420 [Link]
Abstract: Organocatalytic stereoselective addition of different nucleophiles to β-nitroacrylates have been investigated. A thiourea-based cinchona-derived bifunctional catalyst promoted the addition of 1,3-diketones to differently substituted nitroacrylates in modest to fair yields and up to 93% enantioselectivity. The 1,3 dicarbonylic functionality of the addition products was then employed to synthesize enantiomerically enriched 3,4,5-trialkyl substituted pyrazoles via reaction with hydrazine. The stereoselective addition of N-methyl indole to nitro acrylate was also preliminarily studied; the Friedel-Crafts alkylation was successfully organocatalyzed in the presence of an acid additive in very good yields and up to 55% enantioselectivity.