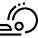Synthesis of pharmaceutical products
A convenient protocol for a zinc-catalysed synthesis of electron-poor indoles
F. Medici, F. Montinari, E. Donato, L. Raimondi, M. Benaglia Tetrahedron Lett , 2023, 116, 154340 [Link]
Abstract: A convenient catalytic protocol for the synthesis of electron-poor indoles was envisioned. A cyclisation reaction of electron- deficient 2-(alkynyl)-anilines, catalysed by Zn(II) salts, was successfully developed and applied to the synthesis of an API precursor. A two-step one-pot procedure starting from aniline derivatives was successfully developed. The use of a readily available, non-noble metal further enhanced the value of the protocol.
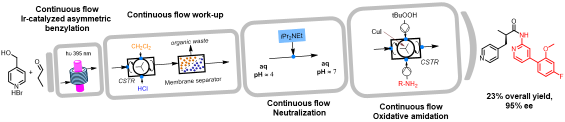
Enantioselective organophotocatalytic telescoped synthesis of a chiral privileged active pharmaceutical ingredient
F. Herbrik , M. Sanz, A. Puglisi, S. Rossi, M. Benaglia Chem. Eur. J., 2022 28 [Link]
Abstract: The continuous flow, enantioselective, organophotoredox catalytic asymmetric alkylation of aldehydes was studied, by using a homemade,
custom designed photoreactor for reactions under cryogenic conditions. Going from microfluidic conditions up to a 10 mL mesofluidic reactor, an increase
of productivity by almost 18000% compared to the batch reaction was demonstrated. Finally, for the first time, a stereoselective photoredox organocatalytic
continuous flow reaction in a fully telescoped process for an API synthesis was successfully achieved. The final process consists of 4 units of operation:
visible light-driven asymmetric catalytic benzylation under continuous flow, inline continuous work-up, neutralisation and a final oxidative amidation step
afforded the pharmaceutically active molecule in 95% e.e.
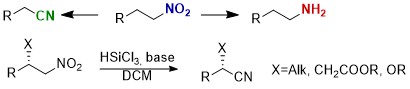
Metal-Free Deoxygenation of Chiral Nitroalkanes: An Easy Entry to α-Substituted Enantiomerically Enriched Nitriles
M. Pirola, C. Faverio, M. Orlandi, M. BenagliaChem. Eur. J. , 2021, 27 10247-10250 [Link]
Abstract: A metal-free, mild and chemodivergent transformation involving nitroalkanes was developed. Under optimized reaction conditions, in the presence of trichlorosilane and a tertiary amine, aliphatic nitroalkanes have been selectively converted into amines or nitriles. Furthermore, when chiral β-substituted nitro compounds were reacted, the stereochemical integrity of the stereocenter was maintained and β-functionalized nitriles were obtained with no loss in enantiomeric excess. The methodology was successfully applied to the synthesis of chiral β-cyano esters, α-aryl alkylnitriles, and TBS-protected cyanohydrins, including direct precursors of four APIs (Ibuprofen, Tembamide, Aegeline and Denopamine).
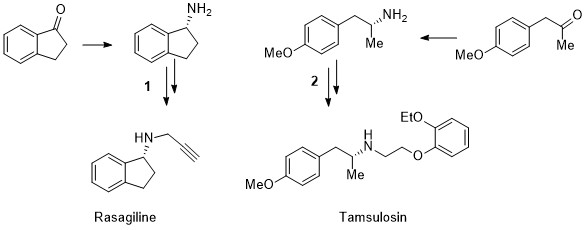
A stereoselective, catalytic strategy for the in-flow synthesis of advanced precursors of Rasagiline and Tamsulosin.
D. Brenna, M. Pirola, L. Raimondi, A. J. Burke, M. Benaglia Bioorg. Med. Chem, 2017, doi:10.1016/j.bmc.2017.01.023 [Link]
Abstract: The diastereoselective, trichlorosilane-mediate reduction of imines, bearing different and removable chiral auxiliaries, in combination either with achiral bases or catalytic amounts of chiral Lewis bases, was investigated to afford immediate precursors of chiral APIs (Active Pharmaceutical Ingredients). The carbon-nitrogen double bond reduction was successfully performed in batch and in flow mode, in high yields and almost complete stereocontrol. By this metal-free approach, the formal synthesis of Rasagiline and Tamsulosin was successfully accomplished in micro(meso) flow reactors, under continuous flow conditions. The results represent a new, important step towards the development of automated processes for the preparation of enantiopure biologically active compounds.
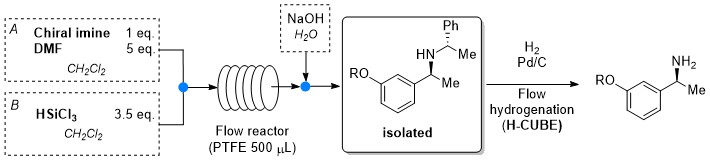
Stereoselective metal-free reduction of chiral imines in batch and in flow mode. A convenient strategy for the synthesis of chiral Active Pharmaceutical Ingredients
D. Brenna, M. Benaglia, R. Porta, S. Fernandes, A. Burke Eur. J. Org. Chem, 2017, 39-44 [Link]
Abstract: A convenient, metal-free reduction of imines, bearing a very cheap and removable chiral auxiliary, allowed the synthesis of immediate precursors of chiral APIs (Active Pharmaceutical Ingredients), in batch and in flow mode, in high yields and almost complete stereocontrol. In the presence of an inexpensive and non toxic reducing agent, like trichlorosilane, and an achiral Lewis base such as N,N-dimethyl formamide, the formal synthesis of Rivastgmine and analogous compounds, calcimimetic NPS R-568 and a Rho kinases inhibitor was successfully accomplished. For the first time, both the diastereoselective imine reduction and the auxiliary removal were efficiently performed in (micro)-mesoreactors under continuous flow conditions, thus paving the way towards a development of a practical process in continuo for the synthesis of industrial relevant, biologically active, enantiopure N-alkyl amines
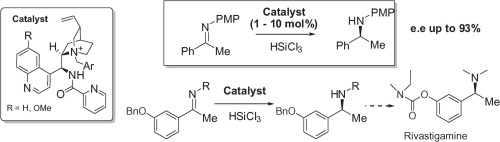
Synthesis of an advanced precursor of Rivastigmine: Cinchona-derived quaternary ammonium salts as organocatalysts for stereoselective imine reductions
A. Genoni, M. Benaglia, E. Mattiolo, S. Rossi, L. Raimondi, P. C. Barrulas, A. J. Burke Tetrahedron. Lett., 2015, 42, 5752-5756[Link]
Abstract:The enantioselective reduction of ketoimines has been successfully realized, using trichlorosilane as the stoichiometric reducing agent in the presence of catalytic amounts of a Lewis base, specifically a Cinchona derivative. For the first time, a novel class of derivatives was studied, featuring a picolinamide unit bound to the alkaloid scaffold, further functionalized as quaternary ammonium salt at the quinuclidine ring. Excellent yields and from good to high enantioselectivities (up to 92% ee) were obtained in the reduction of ketoimines. The novel catalysts were successfully employed in the synthesis of an enantiomerically pure advanced precursor of the blockbuster drug Rivastigmine.
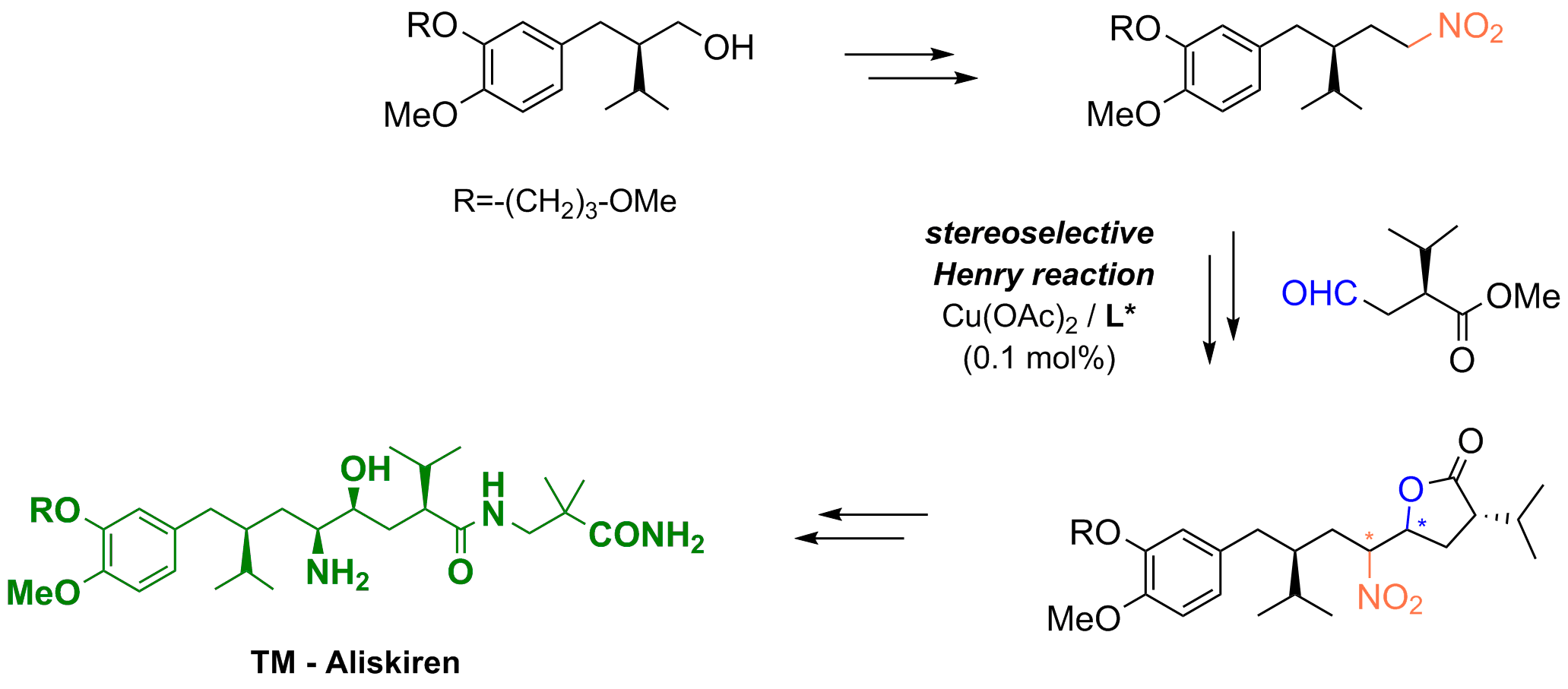
A stereoselective catalytic nitroaldol reaction as key step in a novel strategy for the synthesis of the renin inhibitor Aliskiren
S. Rossi, M. Benaglia, R. Porta, L, Cotarca, P. Maragni, M. Verzini, Eur. J. Org. Chem., 2015, 2531-2537[Link]
Abstract: Aliskiren is the first-in-class orally active direct renin inhibitor. It was approved in 2007 for the treatment of hypertension. We have designed a new strategy for the convergent synthesis of aliskiren that involves a catalytic stereoselective nitroaldol reaction as the key step. A new enantiopure nitroalkane (synthon A1), prepared in only three steps from a commercially available enantiopure 2-(arylmethyl)-3-methyl butanol derivative, was successfully used in a copper-catalysed Henry reaction to give a nitrolactone intermediate in which the correct configuration for the final product was established at all four stereocentres. Nitro-group reduction, Boc-protection of the resulting amine, aminolysis of the lactone with 3-amino-2,2-dimethylpropionamide, and finally Boc-deprotection led to the enantiopure renin inhibitor aliskiren.
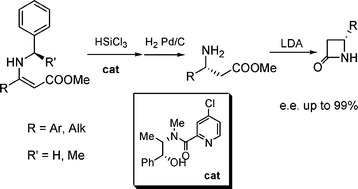
Triclorosilane-mediated stereoselective synthesis of β-amino esters and their conversion to highly enantiomerically enriched β-lactams.
S. Guizzetti, M. Benaglia, M. Bonsignore, L. Raimondi, Org. Biomol. Chem., 2011, 9, 739-743[Link]
Abstract: A highly stereoselective trichlorosilane-mediated reduction of N-benzyl enamines was developed; the combination of a low cost, easy to make metal-free catalyst and an inexpensive chiral auxiliary allowed to perform the reaction on substrates with different structural features often with total control of the stereoselectivity. By easy deprotection through hydrogenolysis followed by conversion of β-aminoester to 2-azetidinones, the synthesis of enantiomerically pure β-lactams (>98% e.e.) was successfully accomplished.