Stereoselective organocatalysis
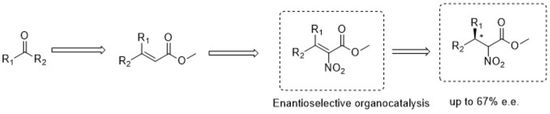
Synthesis of Tetrasubstituted Nitroalkenes and Preliminary Studies of Their Enantioselective Organocatalytic Reduction
P. Camarero Gonzalez, S. Rossi, M. Sanz, F. Vasile, M. Benaglia Molecules 2023, 28, 3156. [Link]
Abstract:
Starting from commercially available ketones, a reproducible and reliable strategy for the synthesis of tetrasubstituted nitroalkenes was successfully developed,
using a two-step procedure; the HWE olefination of the ketone to form the corresponding α,β-unsaturated esters is followed by a nitration reaction to introduce the nitro
group in the α position of the ester group. The enantioselective organocatalytic reduction of these compounds has also been preliminarily studied, to access the functionalized
enantioenriched nitroalkanes, which are useful starting materials for further synthetic elaborations. The absolute configuration of the reduction product was established by
chemical correlation of the chiral nitroalkane with a known product; preliminary DFT calculations were also conducted to rationalize the stereochemical outcome of the
organocatalytic enantioselective reduction.
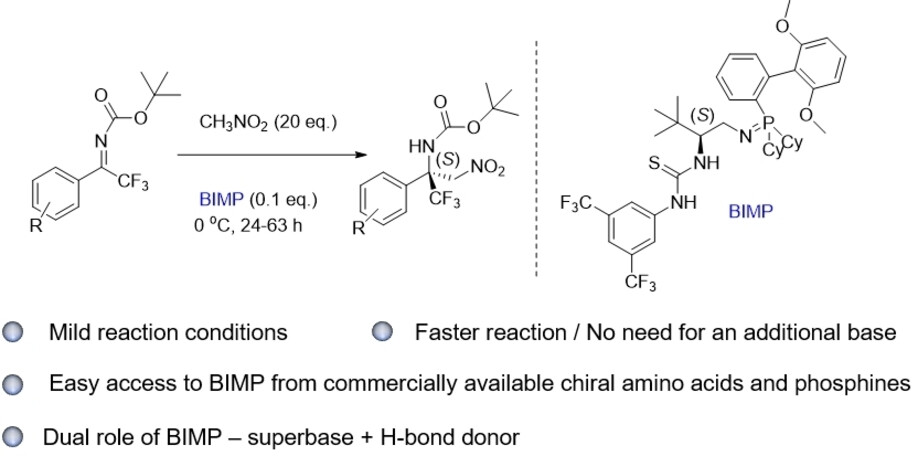
Enantioselective Organocatalytic Addition of Nitromethane to Trifluoromethylaryl Ketoimines Promoted by Electron-Rich Bifunctional Iminophosphoranes
M. Krstić, M. Benaglia, M. Gazzotti, E. Colombo, M. Sanz Adv. Synth. Catal 2023, 24, 365, 1093-1098. [Link]
Abstract:
Thiourea-based iminophosphorane (BIMP) organocatalysts featuring SPhos- or BIDIME phosphine units have been developed and successfully applied in the asymmetric
addition of nitromethane to N-Boc-protected trifluoromethyl aryl ketimines. α-Trifluoromethyl β-nitroamines were obtained in 40–82% isolated yields and 80–95%
enantioselectivities. A careful evaluation of the catalytic activity of BIMPs indicates that the catalysts derived from the combination via Staudinger reaction
of a chiral 1,2-amino alcohol-derived thiourea-organoazide with electron-rich phosphines, promote the aza-Henry reaction on fluorinated ketimines with the highest
enantioselectivity, leading to the amine featuring a tetrasubstituted stereocenter in up to 95% ee. The reaction was performed also on gram scale, without loss of
enantioselectivity.
Catalytic Entry to Enantioenriched Triflones Featuring a Quaternary Stereocenter
F. Franco, S. Meninno, J. Overgaard, S. Rossi, M. Benaglia, A. Lattanzi Org Lett 2022, 24, 24, 4371–4376. [Link]
Abstract: A highly enantioselective one-pot synthesis of functionalised triflones, bearing a quaternary stereocenter, has been developed, exploiting the Michael reaction of α-(trifluoromethylsulfonyl) aryl acetic acid esters with N-acryloyl-1H-pyrazole catalysed by commercially available Takemoto’s catalyst, followed by nucleophilic acyl substitution with alcohols. Preliminary investigations highlighted the attractive potential of the triflinate anion as leaving group for stereocontrolled post-functionalizations.
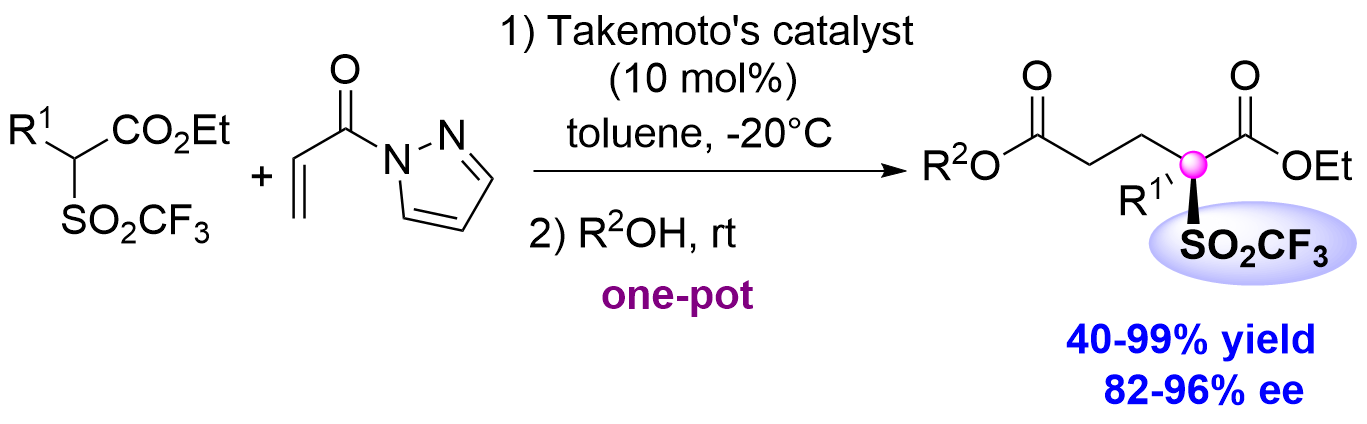
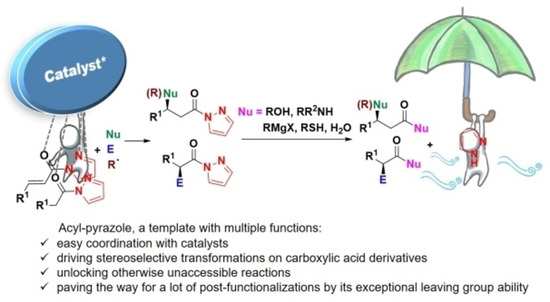
Pyrazoleamides in Catalytic Asymmetric Reactions: Recent Advances
S. Meninno, F. Franco, M. Benaglia, A. Lattanzi Adv. Synth. Catal. 2021, 363, 3380−3410. [Link]
Abstract: Pyrazoleamides are a class of compounds which have received significant attention in the last years in asymmetric catalysis as more reactive and practical surrogates of esters or amides. These readily available reagents have served in a variety of diastereo- and enantioselective metal- and organocatalytic transformations, spanning from simple carbon-carbon or carbon-heteroatom bond formation to cascade and rearrangement reactions, to produce valuable classes of heterocyclic compounds. More recently, pyrazoleamides have proved to be pertinent reagents in asymmetric catalysis merged with photoredox catalysis, and in more challenging stereoselective bond-forming reactions, occurring directly from a visible-light activated substrate/catalyst complex without any charge separation. Both strategies enable new activation concepts useful for the production of difficult-to-access intermediates and pharmaceuticals. In this review, an overview of the progress achieved in asymmetric catalytic reactions of pyrazoleamides from 2015 up to middle 2020, is illustrated.
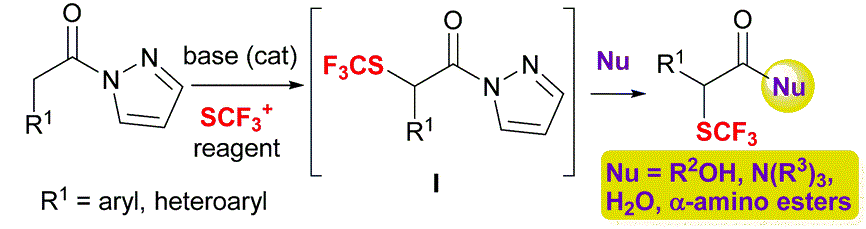
Formal α-trifluoromethylthiolation of carboxylic acid derivatives via N-acyl pyrazoles
F. Franco, S. Mennino, A. Lattanzi, M. Benaglia Chem Comm. 2020, 56, 3073-3076. [Link]
Abstract: Herein we disclosed a one-pot two-step protocol for the first direct, base-catalyzed α-trifluoromethylthiolation of carboxylic acid derivatives by using readily available N-acyl pyrazoles, N-(trifluoromethylthio)phthalimide and a nucleophile such as amines, alcohols and water. Straightforward elaboration of the products to alcohols and triflones expands further the synthetic utility of the process.
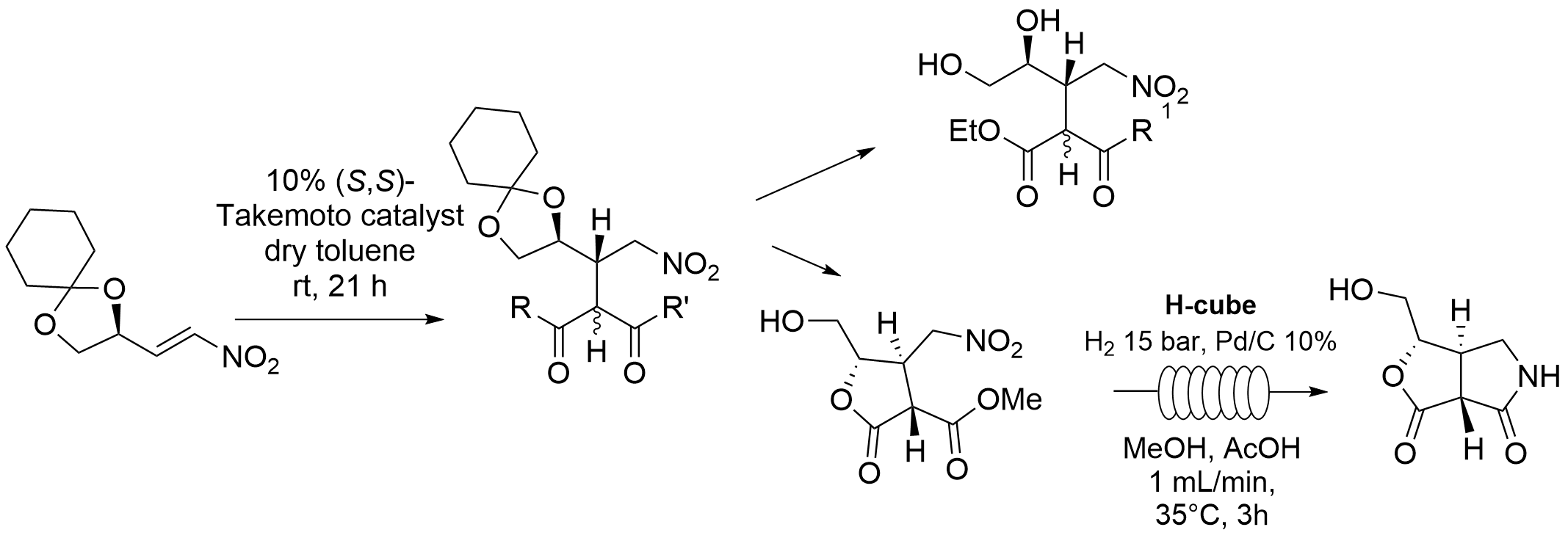
Organocatalytic Michael addition to (D)-mannitol-derived enantiopure nitroalkenes: a valuable strategy for the synthesis of densely functionalized chiral molecules.
L. Caruso, a. Puglisi, E. Gillon, M. Benaglia, molecules, 2019, 24, accepted.
Abstract: (D)-mannitol was selected as valuable platform, directly available from the chiral pool, to build highly functionalized molecules. Starting from (R)-2,3-O-cyclohexylidene glyceraldehyde, easily prepared on large scale from D-mannitol, an enantiopure chiral nitro alkene was prepared, by reaction with nitromethane, and its reactivity studied. Organocatalytic Michael addition of dimethyl malonate, β-keto esters and other nucleophiles on the nitro alkene afforded with high stereoselectivity densely functionalised chiral molecules, which were further synthetically elaborated, leading to five-membered lactones and bicyclic lactams. Preliminary studies showed that the metal-free catalytic reaction on the chiral nitro alkene can be performed also under continuous flow conditions, thus opening the way to the use of (micro)meso fluidic systems for the preparation of enantiomerically pure organic molecules from the chiral pool.
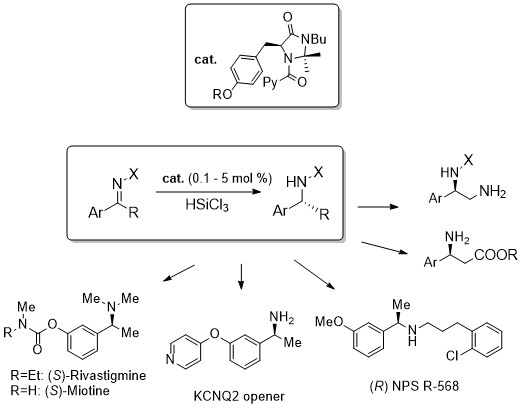
A new class of low-loading catalysts for a highly enantioselective, metal-free imine reduction of wide general applicability
D. Brenna, R. Porta, E. Massolo, L. Raimondi, M. Benaglia ChemCatChem, 2017, 9, 941-945. [Link]
Abstract: A new class of chiral Lewis bases for the enantioselective HSiCl3-mediated reduction of imines has been developed. Through an extensive catalyst structure optimization, an extremely active species was identified, able to promote the reduction of a large variety of functionalized substrates in high yields and enantioselectivities typically above 90% with catalyst loading as low as 0.1-1% mol. The simple experimental procedure, the low cost of the reagents, the mild reaction conditions, and straightforward isolation of the product, make the methodology attractive also for large scale applications. Its synthetic potentiality was demonstrated by the preparation of advanced intermediates of important APIs, used in the treatment of Alzheimer and Parkinson diseases, hyperparathyroidism, neuropathic pains and neurological disorders.The reaction has also been successfully performed in flow reactors, thus demonstrating the possibility to realize in continuo processes yielding multigram quantities of product
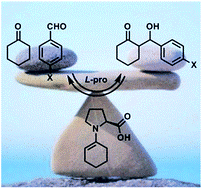
Kinetics versus thermodynamics in the proline catalyzed aldol reaction
M. Orlandi, M. Benaglia, M. Ceotto Chem. Sci., 2016 [Link]
Abstract: In this paper the equilibrium properties of the proline catalyzed aldol reaction was studied. The use of well-established methodologies, like reaction progress kinetic analysis and linear free energy relationship analysis, led to the quantification of the reaction reversibility and to its correlation with the substrate electronic activation. Due to these experimental observations, common computational approaches based on a one way transition state analysis become unsuitable. Therefore, a computational model based on the integration of a system of kinetic differential equations associated to the multiple equilibrium reactions was proposed. Such a model was found to successfully rationalize the chemical and stereochemical outcomes of this paradigmatic reaction for the first time.
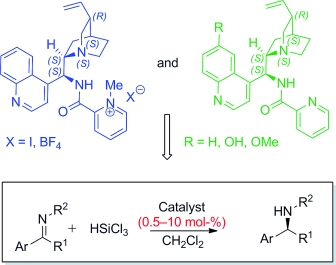
Cinchona-Derived Picolinamides: Effective Organocatalysts for Stereoselective Imine Hydrosilylation
P. C. Barrulas, A. Genoni, M. Benaglia, A. J. Burke Eur. J. Org. Chem., 2014, [Link]
Abstract: Picolinamide-cinchona organocatalysts for the successful enantioselective reduction of ketomines were developed. For the first time, a new type of chiral Lewis base, a cationic species, is reported to efficiently organocatalyze the addition of trichlorosilane to imines. Excellent yields with good to high enantioselectivities (up to 91%) were obtained in the reduction of differently substituted substrates. Noteworthy, remarkably high turnover frequencies for the hydrosilylation of imines were observed; the catalyst of choice proved to be active even at a loading of only 1 mol%. The loading was further reduced to 0.5 mol-%, and for very short reaction times (15 min) very impressive asymmetric catalyst efficiency speed values were reached.
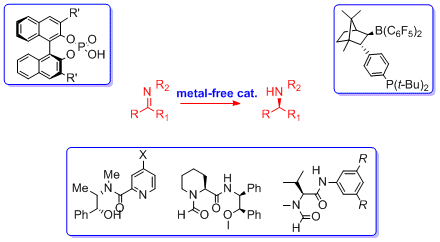
Organocatalytic strategies for enantioselective metal-free reductions
S. Rossi, E. Massolo, L. Raimondi Catal. Sci. Technol., 2014, [Link]
Abstract:One of the most important chemical transformation is the reduction of multiple bonds, carbon-carbon as well as carbon-heteroatom double bonds, since it leads very often to the generation of new stereocenters in the molecule. The replacement of metal-based catalysts with equally efficient metal-free counterparts is very appealing in view of possible applications in the future of non toxic, low cost, and environmentally friendly promoters on industrial scale. The perspective will focus specially, but not exlusively, on the enantioselective reduction of carbon nitrogen double bond; despite the historical need for and continued interest in chiral amines, their synthesis remains challenging. Three metal-free catalytic methodologies available for the reduction of carbon-nitrogen double bond will be discussed: i) binaphthol-derived phosphoric acids catalyzed reductions, with dihydropyridine-based compound as the reducing agent; ii) trichlorosilane mediated reductions, in the presence of catalytic amounts of chiral Lewis bases; iii) metal-free hydrogenation of imines through FLP (Frustrated Lewis Pair) methodology, that involves the use of a combination of the strong Lewis acid with a variety of sterically encumbered Lewis bases, for examples phosphines or tertiary amines, to activate hydrogen at ambient conditions. A special attention will be devoted to the most recent applications of the last five years.
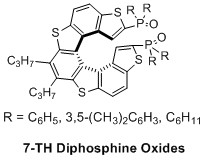
Synthesis, Characterisation, and Organocatalytic Activity of Chiral Tetrathiahelicene Diphosphine Oxides
S. Cauteruccio, D. Dova, M. Benaglia, A. Genoni, M. Orlandi, E. Licandro, Eur. J. Org. Chem., 2014, 13, 2694-2702. [Link]
Abstract: Two new chiral tetrathia[7]helicene (7-TH) based tertiary phosphine oxides (ѩ-2b and (ѩ-2c, bearing two substituents on the phosphorus atoms with different steric and electronic properties, have been synthesised and fully characterised by means of analytical and spectroscopic techniques. The resolution of (ѩ-2aף into their antipodes was accomplished by HPLC separation on a chiral stationary phase, and their chiroptical properties were investigated by CD spectroscopy. The behaviour of 2aף as organocatalysts was assessed in representative reactions mediated by tri- or tetrachlorosilane. 7-TH-based phosphine oxides 2a and 2b promoted both ketoimine reduction and stereoselective carbonףarbon bond formation in good chemical yields and diastereoselectivity, albeit with low enantioselectivity, thus opening the way to the development of a novel class of enantiopure helical-based phosphorus organocatalysts.
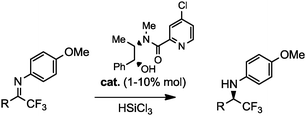
Stereoselective metal-free catalytic synthesis of chiral trifluoromethyl aryl and alkyl amines
A. Genoni, M. Benaglia, E. Massolo and S. Rossi, Chem. Commun., 2013, 49, 8365-8367. [Link]
Abstract: The enantioselective organocatalytic reduction of trifluoromethyl aryl and alkyl ketoimines afforded the corresponding fluorinated amines with high chemical and stereochemical efficiency. The Lewis base catalyzed reaction with trichlorosilane led to chiral products with a trifluoromethyl group directly linked to the newly generated stereocenter typically in >90% yield and up to 98% e.e.
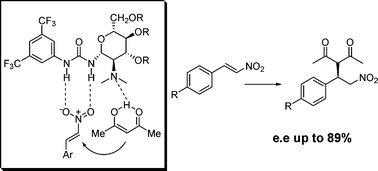
Novel carbohydrate-based bifunctional organocatalysts for nucleophilic addition to nitroolefins and imines.
A. Puglisi, L. Raimondi, M. Benaglia, L. Lay, L. Poletti, Org. Biomol. Chem., 2011, 9, 3295-3302. [Link]
Abstract: Glucosamine has been selected as a cheap and readily available chiral scaffold for the synthesis of a series of novel enantiomerically pure bifunctional organocatalysts bearing a tertiary amino group in proximity to a (thio)urea group. The catalytic behaviour of these compounds, both as neutral and N-protonated species, was investigated using the addition of acetylacetone to β-nitrostyrene as a model reaction. Under optimized experimental conditions, chemical yields up to 93% and enantioselectivities up to 89% were obtained. Semiempirical (AM1) computational studies allowed to find a theoretical rationale for the chemical and stereochemical behaviour of the catalyst of choice. These catalysts were also preliminarily investigated as promoters in the addition of diethyl malonate to the N-Boc imine of benzaldehyde, affording the product in up to 81% ee.